Investigating gene expression profiles of whole blood and peripheral blood mononuclear cells using multiple collection and processing methods
- PMID: 31809517
- PMCID: PMC6897427
- DOI: 10.1371/journal.pone.0225137
Investigating gene expression profiles of whole blood and peripheral blood mononuclear cells using multiple collection and processing methods
Abstract
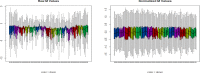
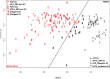
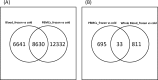
Gene expression profiling using blood samples is a valuable tool for biomarker discovery in clinical studies. Different whole blood RNA collection and processing methods are highly variable and might confound comparisons of results across studies. The main aim of the current study is to compare how blood storage, extraction methodologies, and the blood components themselves may influence gene expression profiling. Whole blood and peripheral blood mononuclear cell (PBMC) samples were collected in triplicate from five healthy donors. Whole blood was collected in RNAgard® and PAXgene® Blood RNA Tubes, as well as in collection tubes with anticoagulants such as dipotassium ethylenediaminetetraacetic acid (K2EDTA) and Acid Citrate Dextrose Solution A (ACD-A). PBMCs were separated using sodium citrate Cell Preparation Tubes (CPT™), FICOLL™, magnetic separation, and the LeukoLOCK™ methods. After blood collection, the LeukoLOCK™, K2EDTA and ACD-A blood tubes were shipped overnight using cold conditions and samples from the rest of the collection were immediately frozen with or without pre-processing. The RNA was isolated from whole blood and PBMCs using a total of 10 different experimental conditions employing several widely utilized RNA isolation methods. The RNA quality was assessed by RNA Integrity Number (RIN), which showed that all PBMC procedures had the highest RIN values when blood was stabilized in TRIzol® Reagent before RNA extraction. Initial data analysis showed that human blood stored and shipped at 4°C overnight performed equally well when checked for quality using RNA integrity number when compared to frozen stabilized blood. Comparisons within and across donor/method replicates showed signal-to-noise patterns which were not captured by RIN value alone. Pathway analysis using the top 1000 false discovery rate (FDR) corrected differentially expressed genes (DEGs) showed frozen vs. cold shipping conditions greatly impacted gene expression patterns in whole blood. However, the top 1000 FDR corrected DEGs from PBMCs preserved after frozen vs. cold shipping conditions (LeukoLOCK™ preserved in RNAlater®) revealed no significantly affected pathways. Our results provide novel insight into how RNA isolation, various storage, handling, and processing methodologies can influence RNA quality and apparent gene expression using blood samples. Careful consideration is necessary to avoid bias resulting from downstream processing. Better characterization of the effects of collection method idiosyncrasies will facilitate further research in understanding the effect of gene expression variability in human sample types.
Conflict of interest statement
The authors are affiliated with the US Army Center for Environmental Health Research. The views, opinions, and/or findings contained in this report are those of the authors and should not be construed as official Department of the Army position, policy, or decision, unless so designated by other official documentation. Citations of commercial organizations or trade names in this report do not constitute an official Department of the Army endorsement or approval of the products or services of these organizations.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

