Ameliorating effects of Gö6976, a pharmacological agent that inhibits protein kinase D, on collagen-induced arthritis
- PMID: 31809526
- PMCID: PMC6897462
- DOI: 10.1371/journal.pone.0226145
Ameliorating effects of Gö6976, a pharmacological agent that inhibits protein kinase D, on collagen-induced arthritis
Abstract
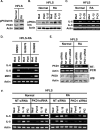
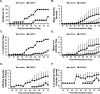
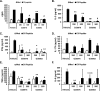
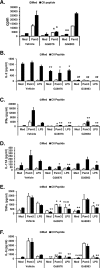
Toll-like receptor (TLR) signaling can contribute to the pathogenesis of arthritis. Disruption of TLR signaling at early stages of arthritis might thereby provide an opportunity to halt the disease progression and ameliorate outcomes. We previously found that Gö6976 inhibits TLR-mediated cytokine production in human and mouse macrophages by inhibiting TLR-dependent activation of protein kinase D1 (PKD1), and that PKD1 is essential for proinflammatory responses mediated by MyD88-dependent TLRs. In this study, we investigated whether PKD1 contributes to TLR-mediated proinflammatory responses in human synovial cells, and whether Gö6976 treatment can suppress the development and progression of type II collagen (CII)-induced arthritis (CIA) in mouse. We found that TLR/IL-1R ligands induced activation of PKD1 in human fibroblast-like synoviocytes (HFLS). TLR/IL-1R-induced expression of cytokines/chemokines was substantially inhibited in Gö6976-treated HFLS and PKD1-knockdown HFLS. In addition, serum levels of anti-CII IgG antibodies, and the incidence and severity of arthritis after CII immunization were significantly reduced in mice treated daily with Gö6976. Synergistic effects of T-cell receptor and TLR, as well as TLR alone, on spleen cell proliferation and cytokine production were significantly inhibited in the presence of Gö6976. Our results suggest a possibility that ameliorating effects of Gö6976 on CIA may be due to its ability to inhibit TLR/IL-1R-activated PKD1, which might play an important role in proinflammatory responses in arthritis, and that PKD1 could be a therapeutic target for inflammatory arthritis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Interleukin-35 (IL-35) inhibits proliferation and promotes apoptosis of fibroblast-like synoviocytes isolated from mice with collagen-induced arthritis.Mol Biol Rep. 2016 Sep;43(9):947-56. doi: 10.1007/s11033-016-4034-7. Epub 2016 Jul 5. Mol Biol Rep. 2016. PMID: 27379996
-
Protection against cartilage and bone destruction by systemic interleukin-4 treatment in established murine type II collagen-induced arthritis.Arthritis Res. 1999;1(1):81-91. doi: 10.1186/ar14. Epub 1999 Oct 26. Arthritis Res. 1999. PMID: 11056663 Free PMC article.
-
PKC-alpha controls MYD88-dependent TLR/IL-1R signaling and cytokine production in mouse and human dendritic cells.Eur J Immunol. 2010 Feb;40(2):505-15. doi: 10.1002/eji.200939391. Eur J Immunol. 2010. PMID: 19950169
-
Hyperoside exerts anti-inflammatory and anti-arthritic effects in LPS-stimulated human fibroblast-like synoviocytes in vitro and in mice with collagen-induced arthritis.Acta Pharmacol Sin. 2016 May;37(5):674-86. doi: 10.1038/aps.2016.7. Epub 2016 Apr 4. Acta Pharmacol Sin. 2016. PMID: 27041460 Free PMC article.
-
Curcumin attenuates inflammatory response in IL-1beta-induced human synovial fibroblasts and collagen-induced arthritis in mouse model.Int Immunopharmacol. 2010 May;10(5):605-10. doi: 10.1016/j.intimp.2010.02.011. Epub 2010 Feb 23. Int Immunopharmacol. 2010. PMID: 20188213
Cited by
-
Protein kinase D1 in myeloid lineage cells contributes to the accumulation of CXCR3+CCR6+ nonconventional Th1 cells in the lungs and potentiates hypersensitivity pneumonitis caused by S. rectivirgula.Front Immunol. 2024 Oct 11;15:1403155. doi: 10.3389/fimmu.2024.1403155. eCollection 2024. Front Immunol. 2024. PMID: 39464896 Free PMC article.
References
-
- van der Heijden IM, Wilbrink B, Tchetverikov I, Schrijver IA, Schouls LM, Hazenberg MP, et al. Presence of bacterial DNA and bacterial peptidoglycans in joints of patients with rheumatoid arthritis and other arthritides. Arthritis Rheum. 2000;43(3):593–8. 10.1002/1529-0131(200003)43:3<593::AID-ANR16>3.0.CO;2-1 . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

