First Insights into the Effect of Low-Dose X-Ray Irradiation in Adipose-Derived Stem Cells
- PMID: 31810198
- PMCID: PMC6928975
- DOI: 10.3390/ijms20236075
First Insights into the Effect of Low-Dose X-Ray Irradiation in Adipose-Derived Stem Cells
Abstract
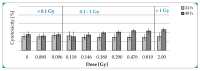
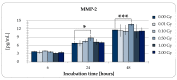
(1) Background: Emerging interest of physicians to use adipose-derived stem cells (ADSCs) for regenerative therapies and the fact that low-dose irradiation (LD-IR ≤ 0.1 Gy) has been reported to enhance the proliferation of several human normal and bone-marrow stem cells, but not that of tumor cells, lead to the idea of improving stem cell therapies via low-dose radiation. Therefore, the aim of this study was to investigate unwanted side effects, as well as proliferation-stimulating mechanisms of LD-IR on ADSCs. (2) Methods: To avoid donor specific effects, ADSCs isolated from mamma reductions of 10 donors were pooled and used for the radiobiological analysis. The clonogenic survival assay was used to classify the long-term effects of low-dose radiation in ADSCs. Afterwards, cytotoxicity and genotoxicity, as well as the effect of irradiation on proliferation of ADSCs were investigated. (3) Results: LD (≤ 0.1 Gy) of ionizing radiation promoted the proliferation and survival of ADSCs. Within this dose range neither geno- nor cytotoxic effects were detectable. In contrast, greater doses within the dose range of >0.1-2.0 Gy induced residual double-strand breaks and reduced the long-term survival, as well as the proliferation rate of ADSCs. (4) Conclusions: Our data suggest that ADSCs are resistant to LD-IR. Furthermore, LD-IR could be a possible mediator to improve approaches of stem cells in the field of regenerative medicine.
Keywords: X-ray; adipose-derived stem cells; low-dose radiation; mesenchymal stem cells; radiation therapy; regenerative medicine.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Richter E., Feyerabend T., Richter E., Feyerabend T. Grundlagen der Strahlentherapie. Springer; Berlin/Heidelberg, Germany: 2012. Strahlentherapie gutartiger Erkrankungen; pp. 396–400. - DOI
-
- Niewald M., Holtmann H., Prokein B., Hautmann M.G., Rösler H.P., Graeber S., Dzierma Y., Ruebe C., Fleckenstein J. Randomized multicenter follow-up trial on the effect of radiotherapy on painful heel spur (plantar fasciitis) comparing two fractionation schedules with uniform total dose: First results after three months’ follow-up. Radiat. Oncol. 2015;10:1–7. doi: 10.1186/s13014-015-0471-z. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

