Immune Cell Infiltration in the Microenvironment of Liver Oligometastasis from Colorectal Cancer: Intratumoural CD8/CD3 Ratio Is a Valuable Prognostic Index for Patients Undergoing Liver Metastasectomy
- PMID: 31810350
- PMCID: PMC6966431
- DOI: 10.3390/cancers11121922
Immune Cell Infiltration in the Microenvironment of Liver Oligometastasis from Colorectal Cancer: Intratumoural CD8/CD3 Ratio Is a Valuable Prognostic Index for Patients Undergoing Liver Metastasectomy
Abstract
: Background: A comprehensive investigation into immune cell infiltration provides more accurate and reliable prognostic information for patients with colorectal liver oligometastases (CLO) after liver metastasectomy.
Methods: Simultaneous detection of the immune constituents CD3+, CD8+, Foxp3+ T, and α-SMA+ cells in the liver oligometastasis of 133 patients was conducted using a four-colour immunohistochemical multiplex technique. Immune cells were quantified, and tumour-infiltrating lymphocyte (TIL) ratios were subsequently calculated. Correlation analysis was performed using Pearson's correlation. Recurrence-free survival (RFS) and overall survival (OS) for TIL ratios were analysed using the Kaplan-Meier method and Cox regression models.
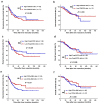
Results: Significantly fewer CD3+, CD8+, and Foxp3+ T cells were observed in the intratumoural region than in the peritumoural region of liver metastases. CD3+, CD8+, Foxp3+ T, and α-SMA+ cells showed significantly positive correlations with each other both in the intratumoural and peritumoural regions of liver metastases. Only the CD8/CD3 TIL ratio demonstrated a positive correlation between intratumoural and peritumoural regions of liver metastases (r = 0.541, p < 0.001). Patients with high intratumoural CD8/CD3 ratios had significantly longer 3-year RFS (59.0% vs. 47.4%, p = 0.035) and 3-year OS rates (83.3% vs. 65.8%, p = 0.007) than those with low intratumoural CD8/CD3 ratios. Multivariate analyses revealed that the intratumoural CD8/CD3 ratio was independently associated with RFS (HR = 0.593; 95% CI = 0.357-0.985; p = 0.043) and OS (HR = 0.391; 95% CI = 0.193-0.794; p = 0.009).
Conclusion: These findings offer a better understanding of the prognostic value of immune cell infiltration on liver oligometastasis from colorectal cancer.
Keywords: CD8/CD3 ratio; colorectal cancer; immune cell; liver oligometastasis; microenvironment.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures







References
-
- Van Cutsem E., Cervantes A., Adam R., Sobrero A., Van Krieken J.H., Aderka D., Aranda Aguilar E., Bardelli A., Benson A., Bodoky G., et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. - DOI - PubMed
-
- Lin J., Peng J., Zhao Y., Luo B., Zhao Y., Deng Y., Sui Q., Gao Y., Zeng Z., Lu Z., et al. Early recurrence in patients undergoing curative resection of colorectal liver oligometastases: Identification of its clinical characteristics, risk factors, and prognosis. J. Cancer Res. Clin. Oncol. 2018;144:359–369. doi: 10.1007/s00432-017-2538-8. - DOI - PMC - PubMed
Grants and funding
- 81772595/National Natural Science Foundation of China
- No. 2015024/Sun Yat-sen University Clinical Research 5010 Program
- No. 2013013/Sun Yat-sen University Clinical Research 5010 Program
- No. 201803040019/Guangzhou Science and Technology Plan Projects
- 2017A030310204/Natural Science Foundation of Guangdong Province
LinkOut - more resources
Full Text Sources
Research Materials

