Validation of an app-based portable spirometer in adolescents with asthma
- PMID: 31810411
- PMCID: PMC7282988
- DOI: 10.1080/02770903.2019.1702201
Validation of an app-based portable spirometer in adolescents with asthma
Abstract
Objectives: Objective measurements of asthma impairment could aid teens in recognition of changes in asthma status over time. Ready access to a conventional spirometer is not realistic outside of the clinical setting. In this proof-of-concept study, we compared the performance of the VitalFlo mobile spirometer to the nSpire KoKo® sx1000 spirometer for accuracy in measuring Forced Expiratory Volume in one second (FEV1) and Forced Vital Capacity (FVC) in adolescents with asthma.
Methods: Two hundred forty pulmonary function measurements were collected from 48 adolescents with persistent asthma from the University of North Carolina's pediatric allergy and pulmonology subspecialty clinics. Participants performed spirometry with the nSpireKoKo® sx1000 spirometer and the VitalFlo spirometer during their clinic visits. 119 simulated FVC maneuvers were conducted on both devices to standardize measurements. Pearson correlations, Bland-Altman procedure, and two-sample comparison tests were performed to assess the relationship between the two spirometers.
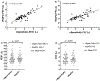
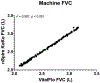
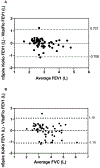
Results: VitalFlo measurements were significantly highly correlated with nSpireKoKo® spirometer values for FEV1, (r2=0.721, [95% CI, 0.749 ± 0.120], P < 0.001) and moderately for FVC (r2= 0.617, [95% CI, 0.640 ± 0.130], P < 0.001) measurements. There were no statistically significant differences of the mean FEV1 (M = 0.00764, SD = 0.364, t(59)=0.16, P = 0.87) and FVC measurements (M = 0.00261, SD = 0.565, t(59)=0.036, P = 0.97.) between the VitalFlo and nSpireKoKo® systems. Both devices demonstrated significantly high correlation when comparing the automated FVC (r2 = 0.997, [95% CI, 1.00 ± 0.00974], P < 0.001) measurements. Bland-Altman plots did not demonstrate significant bias between devices for both FEV1 (0.00764 L) and FVC (0.00261 L) measurements.
Conclusions: Lung function measurements from the VitalFlo mobile spirometer were comparable to a commercially-available spirometer commonly used in clinical settings. This validated app-based spirometer for home use has the potential to improve asthma self-management.
Keywords: Asthma; NCT02662413; NCT02671643; adolescent; bronchial diseases; control; immediate hypersensitivity; obstructive lung diseases; respiratory hypersensitivity; respiratory tract disease.
Figures
Similar articles
-
The utility of hand-held mobile spirometer technology in a resource-constrained setting.S Afr Med J. 2019 Mar 29;109(4):219-222. doi: 10.7196/SAMJ.2019.v109i4.13845. S Afr Med J. 2019. PMID: 31084684
-
Reliability and usability of a portable spirometer compared to a laboratory spirometer.BMC Pulm Med. 2025 May 10;25(1):228. doi: 10.1186/s12890-025-03690-1. BMC Pulm Med. 2025. PMID: 40349062 Free PMC article. Clinical Trial.
-
Comparison of a portable, pneumotach flow-sensor-based spirometer (Spirofy™) with the vitalograph alpha Touch™ spirometer in evaluating lung function in healthy individuals, asthmatics, and COPD patients-a randomized, crossover study.BMC Pulm Med. 2024 May 10;24(1):230. doi: 10.1186/s12890-024-02972-4. BMC Pulm Med. 2024. PMID: 38730359 Free PMC article. Clinical Trial.
-
A Review of Portable Electronic Spirometers: Implications for Asthma Self-Management.Curr Allergy Asthma Rep. 2018 Aug 25;18(10):53. doi: 10.1007/s11882-018-0809-3. Curr Allergy Asthma Rep. 2018. PMID: 30145683
-
Enhancing Adult Asthma Management: A Review on the Utility of Remote Home Spirometry and Mobile Applications.J Pers Med. 2024 Aug 11;14(8):852. doi: 10.3390/jpm14080852. J Pers Med. 2024. PMID: 39202043 Free PMC article. Review.
Cited by
-
Clinical Validation of the Spirohome Clinic Ultrasonic Spirometer in Child and Adolescent Patients.J Asthma Allergy. 2022 Feb 15;15:219-229. doi: 10.2147/JAA.S345189. eCollection 2022. J Asthma Allergy. 2022. PMID: 35210788 Free PMC article.
-
Validation of a new portable system containing both FeNO analysis and spirometry measurement.Front Med (Lausanne). 2023 Aug 31;10:1210329. doi: 10.3389/fmed.2023.1210329. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37720515 Free PMC article.
-
Addressing Reduced Laboratory-Based Pulmonary Function Testing During a Pandemic.Chest. 2020 Dec;158(6):2502-2510. doi: 10.1016/j.chest.2020.06.065. Epub 2020 Jul 8. Chest. 2020. PMID: 32652095 Free PMC article. Review.
-
[Telemedicine and severe asthma in our environment: Views on the experience of professionals and suggestions to make it a reality].Open Respir Arch. 2023 Mar 17;5(3):100239. doi: 10.1016/j.opresp.2023.100239. eCollection 2023 Jul-Sep. Open Respir Arch. 2023. PMID: 37810420 Free PMC article. Review. Spanish.
-
The effect of a systematic multi-dimensional assessment in severe uncontrolled asthma: a literature review and protocol for an investigator-initiated, open-label, randomized-controlled trial (EXACT@home study).BMC Pulm Med. 2025 May 17;25(1):240. doi: 10.1186/s12890-025-03646-5. BMC Pulm Med. 2025. PMID: 40382637 Free PMC article. Review.
References
-
- Nurmagambetov T, Kuwahara R, and Garbe P, The economic burden of asthma in the United States, 2008–2013. Annals of the American Thoracic Society, 2018. 15(3): p. 348–356. - PubMed
-
- Horak E, et al., Lung function and symptom perception in children with asthma and their parents. Pediatric pulmonology, 2003. 35(1): p. 23–28. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
