Insights on the Conformational Ensemble of Cyt C Reveal a Compact State during Peroxidase Activity
- PMID: 31810655
- PMCID: PMC6950772
- DOI: 10.1016/j.bpj.2019.11.011
Insights on the Conformational Ensemble of Cyt C Reveal a Compact State during Peroxidase Activity
Abstract
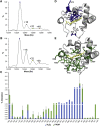
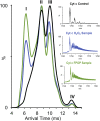
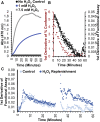
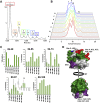
Cytochrome c (cyt c) is known for its role in the electron transport chain but transitions to a peroxidase-active state upon exposure to oxidative species. The peroxidase activity ultimately results in the release of cyt c into the cytosol for the engagement of apoptosis. The accumulation of oxidative modifications that accompany the onset of the peroxidase function are well-characterized. However, the concurrent structural and conformational transitions of cyt c remain undercharacterized. Fast photochemical oxidation of proteins (FPOP) coupled with mass spectrometry is a protein footprinting technique used to structurally characterize proteins. FPOP coupled with native ion mobility separation shows that exposure to H2O2 results in the accumulation of a compact state of cyt c. Subsequent top-down fragmentation to localize FPOP modifications reveals changes in heme coordination between conformers. A time-resolved functional assay suggests that this compact conformer is peroxidase active. Altogether, combining FPOP, ion mobility separation, and top-down and bottom-up mass spectrometry allows us to discern individual conformations in solution and obtain a better understanding of the conformational ensemble and structural transitions of cyt c as it transitions from a respiratory role to a proapoptotic role.
Copyright © 2019 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Jiang X., Wang X. Cytochrome C-mediated apoptosis. Annu. Rev. Biochem. 2004;73:87–106. - PubMed
-
- Ow Y.P., Green D.R., Mak T.W. Cytochrome c: functions beyond respiration. Nat. Rev. Mol. Cell Biol. 2008;9:532–542. - PubMed
-
- Li P., Nijhawan D., Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. - PubMed
-
- Zou H., Li Y., Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem. 1999;274:11549–11556. - PubMed
-
- Platoshyn O., Zhang S., Yuan J.X. Cytochrome c activates K+ channels before inducing apoptosis. Am. J. Physiol. Cell Physiol. 2002;283:C1298–C1305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

