Stem, Effector, and Hybrid States of Memory CD8+ T Cells
- PMID: 31810790
- PMCID: PMC6934921
- DOI: 10.1016/j.it.2019.11.004
Stem, Effector, and Hybrid States of Memory CD8+ T Cells
Abstract
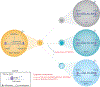
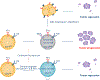
CD8+ T cell immunological memory of past antigen exposure can confer long-lived protection against infections or tumors. The fact that CD8+ memory T cells can have features of both naïve and effector cells has forced the field to struggle with several conceptual questions about the developmental origin of the cell and, consequently, the mechanism(s) that contribute to memory development. Here, we discuss recent conceptual advances in our understanding of memory T cell development that incorporate data describing a hybrid stem and/or effector state of differentiation. We theorize that the mechanisms involved in developing these cells could be mediated, in part, through epigenetic programs. Finally, we consider the potential therapeutic implications of inducing and/or utilizing such hybrid cells clinically.
Keywords: CD8+ T cells; TCF1; cancer immunotherapy; effector; exhaustion; memory; naïve.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Competing interests.
The authors declare no competing financial interests.
Figures

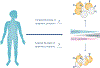
References
-
- Kaech SM et al. (2003) Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol 4 (12), 1191–8. - PubMed
-
- Mahnke YD et al. (2013) The who’s who of T-cell differentiation: Human memory T-cell subsets. Eur J Immunol 43 (11), 2797–809. - PubMed
-
- Kaech SM et al. (2002) Molecular and functional profiling of memory CD8 T cell differentiation. Cell 111 (6), 837–51. - PubMed
-
- Intlekofer AM et al. (2005) Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol 6 (12), 1236–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

