Vasomotion as a Driving Force for Paravascular Clearance in the Awake Mouse Brain
- PMID: 31810839
- PMCID: PMC7028316
- DOI: 10.1016/j.neuron.2019.10.033
Vasomotion as a Driving Force for Paravascular Clearance in the Awake Mouse Brain
Abstract
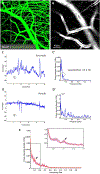
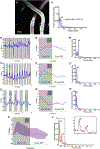
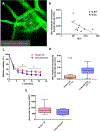
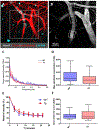
Paravascular drainage of solutes, including β-amyloid (Aβ), appears to be an important process in brain health and diseases such as Alzheimer's disease (AD) and cerebral amyloid angiopathy (CAA). However, the major driving force for clearance remains largely unknown. Here we used in vivo two-photon microscopy in awake head-fixed mice to assess the role of spontaneous vasomotion in paravascular clearance. Vasomotion correlated with paravascular clearance of fluorescent dextran from the interstitial fluid. Increasing the amplitude of vasomotion by means of visually evoked vascular responses resulted in increased clearance rates in the visual cortex of awake mice. Evoked vascular reactivity was impaired in mice with CAA, which corresponded to slower clearance rates. Our findings suggest that low-frequency arteriolar oscillations drive drainage of solutes. Targeting naturally occurring vasomotion in patients with CAA or AD may be a promising early therapeutic option for prevention of Aβ accumulation in the brain.
Keywords: Alzheimer’s disease; cerebral amyloid angiopathy; functional hyperemia; glymphatics; intra-mural peri-arterial drainage; perivascular space; vascular smooth muscle cells; vasomotion.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures


Comment in
-
Vasomotion Drives Periarterial Drainage of Aβ from the Brain.Neuron. 2020 Feb 5;105(3):400-401. doi: 10.1016/j.neuron.2020.01.011. Neuron. 2020. PMID: 32027828
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

