T cell-derived interferon-γ programs stem cell death in immune-mediated intestinal damage
- PMID: 31811055
- PMCID: PMC7239329
- DOI: 10.1126/sciimmunol.aay8556
T cell-derived interferon-γ programs stem cell death in immune-mediated intestinal damage
Abstract
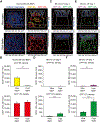
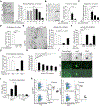
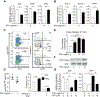
Despite the importance of intestinal stem cells (ISCs) for epithelial maintenance, there is limited understanding of how immune-mediated damage affects ISCs and their niche. We found that stem cell compartment injury is a shared feature of both alloreactive and autoreactive intestinal immunopathology, reducing ISCs and impairing their recovery in T cell-mediated injury models. Although imaging revealed few T cells near the stem cell compartment in healthy mice, donor T cells infiltrating the intestinal mucosa after allogeneic bone marrow transplantation (BMT) primarily localized to the crypt region lamina propria. Further modeling with ex vivo epithelial cultures indicated ISC depletion and impaired human as well as murine organoid survival upon coculture with activated T cells, and screening of effector pathways identified interferon-γ (IFNγ) as a principal mediator of ISC compartment damage. IFNγ induced JAK1- and STAT1-dependent toxicity, initiating a proapoptotic gene expression program and stem cell death. BMT with IFNγ-deficient donor T cells, with recipients lacking the IFNγ receptor (IFNγR) specifically in the intestinal epithelium, and with pharmacologic inhibition of JAK signaling all resulted in protection of the stem cell compartment. In addition, epithelial cultures with Paneth cell-deficient organoids, IFNγR-deficient Paneth cells, IFNγR-deficient ISCs, and purified stem cell colonies all indicated direct targeting of the ISCs that was not dependent on injury to the Paneth cell niche. Dysregulated T cell activation and IFNγ production are thus potent mediators of ISC injury, and blockade of JAK/STAT signaling within target tissue stem cells can prevent this T cell-mediated pathology.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures
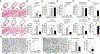


Comment in
-
IFN-γ: The T cell's license to kill stem cells in the inflamed intestine.Sci Immunol. 2019 Dec 6;4(42):eaaz6821. doi: 10.1126/sciimmunol.aaz6821. Sci Immunol. 2019. PMID: 31811056
References
-
- Gehart H, Clevers H, Repairing organs: lessons from intestine and liver. Trends Genet 31, 344–351 (2015). - PubMed
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H, Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007). - PubMed
-
- Clevers H, The intestinal crypt, a prototype stem cell compartment. Cell 154, 274–284 (2013). - PubMed
-
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H, Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6, 25–36 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

