Engineering cytochrome P450 enzyme systems for biomedical and biotechnological applications
- PMID: 31811088
- PMCID: PMC6970918
- DOI: 10.1074/jbc.REV119.008758
Engineering cytochrome P450 enzyme systems for biomedical and biotechnological applications
Abstract
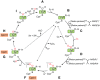
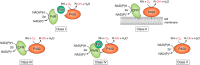
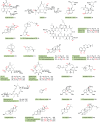
Cytochrome P450 enzymes (P450s) are broadly distributed among living organisms and play crucial roles in natural product biosynthesis, degradation of xenobiotics, steroid biosynthesis, and drug metabolism. P450s are considered as the most versatile biocatalysts in nature because of the vast variety of substrate structures and the types of reactions they catalyze. In particular, P450s can catalyze regio- and stereoselective oxidations of nonactivated C-H bonds in complex organic molecules under mild conditions, making P450s useful biocatalysts in the production of commodity pharmaceuticals, fine or bulk chemicals, bioremediation agents, flavors, and fragrances. Major efforts have been made in engineering improved P450 systems that overcome the inherent limitations of the native enzymes. In this review, we focus on recent progress of different strategies, including protein engineering, redox-partner engineering, substrate engineering, electron source engineering, and P450-mediated metabolic engineering, in efforts to more efficiently produce pharmaceuticals and other chemicals. We also discuss future opportunities for engineering and applications of the P450 systems.
Keywords: cytochrome P450; directed evolution; electron source engineering; enzyme mechanism; ferredoxin; light activation; metabolic engineering; protein engineering; redox partner proteins; substrate engineering; substrate specificity.
© 2020 Li et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

