Mitigation of acyl-homoserine lactone (AHL) based bacterial quorum sensing, virulence functions, and biofilm formation by yttrium oxide core/shell nanospheres: Novel approach to combat drug resistance
- PMID: 31811221
- PMCID: PMC6898131
- DOI: 10.1038/s41598-019-53920-w
Mitigation of acyl-homoserine lactone (AHL) based bacterial quorum sensing, virulence functions, and biofilm formation by yttrium oxide core/shell nanospheres: Novel approach to combat drug resistance
Abstract
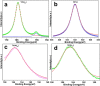
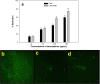
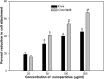
The present study evaluated the efficacy of Y2O3:Tb (core) and Y2O3:Tb@SiO2 nanospheres (core/shell NSs) against virulence functions regulated by quorum sensing (QS) and biofilm formation in pathogenic bacteria. Scanning electron microscope (SEM) images were used to study the size, shape, and morphology. The images clearly displayed spherical shaped, mono-dispersed particles with narrow size distribution and an average grain size of 110-130 nm. The chemical composition of the samples was determined by using energy dispersive X-ray (EDX) and X-ray photoelectron spectroscopy (XPS). We determined the impact of core and core/shell NSs on QS using sensor strains of Chromobacterium violaceum CVO26 and Pseudomonas aeruginosa PAO1 in a comparative study. Sub-MICs of core and core/shell NSs substantially suppressed QS-controlled violacein production in C. violaceum. Similar concentration-dependent effect of sub-MICs of synthesized core and core/shell NSs was observed in the QS-regulated virulence functions (elastase, total protease, pyocyanin production, swarming motility, and exopolysaccharide production) in PAO1. A concentration-dependent decrease (14-60%) was recorded in the biofilm forming capability of PAO1, upon treatment with core and core/shell NSs. Moreover, core/shell NSs were more effective in inhibiting biofilm at higher tested concentrations as compared to core-NSs. The synthesized NSs demonstrated significantly impaired attachment of cells to the microtiter plate indicating that NSs target biofilm inhibition at the attachment stage. Based on these results, we predict that core and core/shell NSs may be an alternative to combat the threat of drug-resistant pathogenic bacteria.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Evaluation of anti-quorum-sensing activity of edible plants and fruits through inhibition of the N-acyl-homoserine lactone system in Chromobacterium violaceum and Pseudomonas aeruginosa.Chemotherapy. 2010;56(4):333-9. doi: 10.1159/000320185. Epub 2010 Aug 18. Chemotherapy. 2010. PMID: 20720417
-
Anti-quorum sensing activity of Psidium guajava L. flavonoids against Chromobacterium violaceum and Pseudomonas aeruginosa PAO1.Microbiol Immunol. 2014 May;58(5):286-93. doi: 10.1111/1348-0421.12150. Microbiol Immunol. 2014. PMID: 24698116
-
Doxycycline interferes with quorum sensing-mediated virulence factors and biofilm formation in gram-negative bacteria.World J Microbiol Biotechnol. 2013 Jun;29(6):949-57. doi: 10.1007/s11274-013-1252-1. Epub 2013 Jan 9. World J Microbiol Biotechnol. 2013. PMID: 23299903
-
Seed Extract of Psoralea corylifolia and Its Constituent Bakuchiol Impairs AHL-Based Quorum Sensing and Biofilm Formation in Food- and Human-Related Pathogens.Front Cell Infect Microbiol. 2018 Oct 25;8:351. doi: 10.3389/fcimb.2018.00351. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30410871 Free PMC article.
-
AHL-Based Quorum Sensing Regulates the Biosynthesis of a Variety of Bioactive Molecules in Bacteria.J Nat Prod. 2024 Apr 26;87(4):1268-1284. doi: 10.1021/acs.jnatprod.3c00672. Epub 2024 Feb 23. J Nat Prod. 2024. PMID: 38390739 Review.
Cited by
-
Anti-QS Strategies Against Pseudomonas aeruginosa Infections.Microorganisms. 2025 Aug 7;13(8):1838. doi: 10.3390/microorganisms13081838. Microorganisms. 2025. PMID: 40871342 Free PMC article. Review.
-
Nanomaterials Regulate Bacterial Quorum Sensing: Applications, Mechanisms, and Optimization Strategies.Adv Sci (Weinh). 2024 Apr;11(15):e2306070. doi: 10.1002/advs.202306070. Epub 2024 Feb 13. Adv Sci (Weinh). 2024. PMID: 38350718 Free PMC article. Review.
-
Microwave-Assisted Rapid Green Synthesis of Gold Nanoparticles Using Seed Extract of Trachyspermum ammi: ROS Mediated Biofilm Inhibition and Anticancer Activity.Biomolecules. 2021 Jan 30;11(2):197. doi: 10.3390/biom11020197. Biomolecules. 2021. PMID: 33573343 Free PMC article.
-
Metal-Based Approaches for the Fight against Antimicrobial Resistance: Mechanisms, Opportunities, and Challenges.J Am Chem Soc. 2025 Apr 16;147(15):12361-12380. doi: 10.1021/jacs.4c16035. Epub 2025 Mar 10. J Am Chem Soc. 2025. PMID: 40063057 Free PMC article. Review.
-
Quorum Sensing Inhibitors: An Alternative Strategy to Win the Battle against Multidrug-Resistant (MDR) Bacteria.Molecules. 2024 Jul 24;29(15):3466. doi: 10.3390/molecules29153466. Molecules. 2024. PMID: 39124871 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

