Transferrin plays a central role in coagulation balance by interacting with clotting factors
- PMID: 31811276
- PMCID: PMC7015052
- DOI: 10.1038/s41422-019-0260-6
Transferrin plays a central role in coagulation balance by interacting with clotting factors
Abstract
Coagulation balance is maintained through fine-tuned interactions among clotting factors, whose physiological concentrations vary substantially. In particular, the concentrations of coagulation proteases (pM to nM) are much lower than their natural inactivator antithrombin (AT, ~ 3 μM), suggesting the existence of other coordinators. In the current study, we found that transferrin (normal plasma concentration ~40 μM) interacts with fibrinogen, thrombin, factor XIIa (FXIIa), and AT with different affinity to maintain coagulation balance. Normally, transferrin is sequestered by binding with fibrinogen (normal plasma concentration ~10 μM) at a molar ratio of 4:1. In atherosclerosis, abnormally up-regulated transferrin interacts with and potentiates thrombin/FXIIa and blocks AT's inactivation effect on coagulation proteases by binding to AT, thus inducing hypercoagulability. In the mouse model, transferrin overexpression aggravated atherosclerosis, whereas transferrin inhibition via shRNA knockdown or treatment with anti-transferrin antibody or designed peptides interfering with transferrin-thrombin/FXIIa interactions alleviated atherosclerosis. Collectively, these findings identify that transferrin is an important clotting regulator and an adjuster in the maintenance of coagulation balance and modifies the coagulation cascade.
Conflict of interest statement
The authors declare no competing interests.
Figures



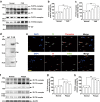


Comment in
-
Transferrin: a blood coagulation modifier.Cell Res. 2020 Feb;30(2):101-102. doi: 10.1038/s41422-020-0275-z. Cell Res. 2020. PMID: 31953530 Free PMC article. No abstract available.
Similar articles
-
Iron-Deficiency and Estrogen Are Associated With Ischemic Stroke by Up-Regulating Transferrin to Induce Hypercoagulability.Circ Res. 2020 Aug 14;127(5):651-663. doi: 10.1161/CIRCRESAHA.119.316453. Epub 2020 May 26. Circ Res. 2020. PMID: 32450779
-
Hypoxia and low temperature upregulate transferrin to induce hypercoagulability at high altitude.Blood. 2022 Nov 10;140(19):2063-2075. doi: 10.1182/blood.2022016410. Blood. 2022. PMID: 36040436 Free PMC article.
-
Systems biology of coagulation initiation: kinetics of thrombin generation in resting and activated human blood.PLoS Comput Biol. 2010 Sep 30;6(9):e1000950. doi: 10.1371/journal.pcbi.1000950. PLoS Comput Biol. 2010. PMID: 20941387 Free PMC article.
-
Formation of the fibrin clot: the balance of procoagulant and inhibitory factors.Clin Haematol. 1985 Jun;14(2):281-342. Clin Haematol. 1985. PMID: 2994929 Review.
-
The Coagulation Factors Fibrinogen, Thrombin, and Factor XII in Inflammatory Disorders-A Systematic Review.Front Immunol. 2018 Jul 26;9:1731. doi: 10.3389/fimmu.2018.01731. eCollection 2018. Front Immunol. 2018. PMID: 30105021 Free PMC article.
Cited by
-
Albumin, oral contraceptives, and venous thromboembolism risk in astronauts.J Appl Physiol (1985). 2022 May 1;132(5):1232-1239. doi: 10.1152/japplphysiol.00024.2022. Epub 2022 Apr 7. J Appl Physiol (1985). 2022. PMID: 35389755 Free PMC article.
-
Progress of Ferroptosis in Ischemic Stroke and Therapeutic Targets.Cell Mol Neurobiol. 2024 Feb 23;44(1):25. doi: 10.1007/s10571-024-01457-6. Cell Mol Neurobiol. 2024. PMID: 38393376 Free PMC article. Review.
-
Elevated Transferrin at High Altitude: Trick or Treat?Hemasphere. 2023 Feb 23;7(3):e850. doi: 10.1097/HS9.0000000000000850. eCollection 2023 Mar. Hemasphere. 2023. PMID: 36844187 Free PMC article. No abstract available.
-
Transferrin Is Up-Regulated by Microbes and Acts as a Negative Regulator of Immunity to Induce Intestinal Immunotolerance.Research (Wash D C). 2024 Jan 25;7:0301. doi: 10.34133/research.0301. eCollection 2024. Research (Wash D C). 2024. PMID: 38274126 Free PMC article.
-
ShSPI Inhibits Thrombosis Formation and Ischemic Stroke In Vivo.Int J Mol Sci. 2024 Aug 19;25(16):9003. doi: 10.3390/ijms25169003. Int J Mol Sci. 2024. PMID: 39201690 Free PMC article.
References
-
- Liuzzo G. Atherosclerosis: an inflammatory disease. Rays. 2001;26:221–230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

