Role of BDNF in the pathophysiology and treatment of depression: Activity-dependent effects distinguish rapid-acting antidepressants
- PMID: 31811669
- PMCID: PMC7274898
- DOI: 10.1111/ejn.14630
Role of BDNF in the pathophysiology and treatment of depression: Activity-dependent effects distinguish rapid-acting antidepressants
Abstract
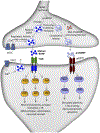
The pathophysiology and treatment of depression have been the focus of intense research and while there is much that remains unknown, modern neurobiological approaches are making progress. This work demonstrates that stress and depression are associated with atrophy of neurons and reduced synaptic connectivity in brain regions such as the hippocampus and prefrontal cortex that contribute to depressive behaviors, and conversely that antidepressant treatment can reverse these deficits. The role of neurotrophic factors, particularly brain-derived neurotrophic factor (BDNF), has been of particular interest as these factors play a key role in activity-dependent regulation of synaptic plasticity. Here, we review the literature demonstrating that exposure to stress and depression decreases BDNF expression in the hippocampus and PFC and conversely that antidepressant treatment can up-regulate BDNF in the adult brain and reverse the effects of stress. We then focus on rapid-acting antidepressants, particularly the NMDA receptor antagonist ketamine, which produces rapid synaptic and antidepressant behavioral actions that are dependent on activity-dependent release of BDNF. This rapid release of BDNF differs from typical monoaminergic agents that require chronic administration to produce a slow induction of BDNF expression, consistent with the time lag for the therapeutic action of these agents. We review evidence that other classes of rapid-acting agents also require BDNF release, demonstrating that this is a common, convergent downstream mechanism. Finally, we discuss evidence that the actions of ketamine are also dependent on another growth factor, vascular endothelial growth factor (VEGF) and its complex interplay with BDNF.
Keywords: ketamine; neurotrophic factor; stress; synaptic plasticity.
© 2019 Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
Figures

Similar articles
-
Neurotrophic mechanisms underlying the rapid and sustained antidepressant actions of ketamine.Pharmacol Biochem Behav. 2020 Jan;188:172837. doi: 10.1016/j.pbb.2019.172837. Epub 2019 Dec 9. Pharmacol Biochem Behav. 2020. PMID: 31830487 Free PMC article. Review.
-
Role of neurotrophic and growth factors in the rapid and sustained antidepressant actions of ketamine.Neuropharmacology. 2023 Feb 15;224:109335. doi: 10.1016/j.neuropharm.2022.109335. Epub 2022 Nov 17. Neuropharmacology. 2023. PMID: 36403852 Review.
-
Activity-dependent brain-derived neurotrophic factor signaling is required for the antidepressant actions of (2R,6R)-hydroxynorketamine.Proc Natl Acad Sci U S A. 2019 Jan 2;116(1):297-302. doi: 10.1073/pnas.1814709116. Epub 2018 Dec 17. Proc Natl Acad Sci U S A. 2019. PMID: 30559184 Free PMC article.
-
Neurotrophic and Antidepressant Actions of Brain-Derived Neurotrophic Factor Require Vascular Endothelial Growth Factor.Biol Psychiatry. 2019 Jul 15;86(2):143-152. doi: 10.1016/j.biopsych.2018.12.014. Epub 2018 Dec 27. Biol Psychiatry. 2019. PMID: 30712809 Free PMC article.
-
BDNF - a key transducer of antidepressant effects.Neuropharmacology. 2016 Mar;102:72-9. doi: 10.1016/j.neuropharm.2015.10.034. Epub 2015 Nov 11. Neuropharmacology. 2016. PMID: 26519901 Free PMC article. Review.
Cited by
-
Synaptotagmin-4 induces anhedonic responses to chronic stress via BDNF signaling in the medial prefrontal cortex.Exp Mol Med. 2024 Feb;56(2):329-343. doi: 10.1038/s12276-024-01156-8. Epub 2024 Feb 1. Exp Mol Med. 2024. PMID: 38297157 Free PMC article.
-
Unhealthy Lifestyle and Gut Dysbiosis: A Better Understanding of the Effects of Poor Diet and Nicotine on the Intestinal Microbiome.Front Endocrinol (Lausanne). 2021 Jun 8;12:667066. doi: 10.3389/fendo.2021.667066. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34168615 Free PMC article. Review.
-
BDNF Gene Polymorphism and Antidepressant Response in Han Chinese Patients with First-Episode Late-Life Depression.Alpha Psychiatry. 2025 Apr 24;26(2):39955. doi: 10.31083/AP39955. eCollection 2025 Apr. Alpha Psychiatry. 2025. PMID: 40352065 Free PMC article.
-
Gene Expression After Exercise Is Disrupted by Early-Life Stress.Dev Psychobiol. 2025 Jan;67(1):e70017. doi: 10.1002/dev.70017. Dev Psychobiol. 2025. PMID: 39780028 Free PMC article.
-
Chronic Trazodone and Citalopram Treatments Increase Trophic Factor and Circadian Rhythm Gene Expression in Rat Brain Regions Relevant for Antidepressant Efficacy.Int J Mol Sci. 2022 Nov 14;23(22):14041. doi: 10.3390/ijms232214041. Int J Mol Sci. 2022. PMID: 36430520 Free PMC article.
References
-
- Advani T, Koek W & Hensler JG (2009) Gender differences in the enhanced vulnerability of BDNF+/− mice to mild stress. Int J Neuropsychopharmacol, 12, 583–588. - PubMed
-
- Antila H, Ryazantseva M, Popova D, Sipila P, Guirado R, Kohtala S, Yalcin I, Lindholm J, Vesa L, Sato V, Cordeira J, Autio H, Kislin M, Rios M, Joca S, Casarotto P, Khiroug L, Lauri S, Taira T, Castren E & Rantamaki T (2017) Isoflurane produces antidepressant effects and induces TrkB signaling in rodents. Sci Rep, 7, 7811. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

