Susceptibility of larval zebrafish to the seizurogenic activity of GABA type A receptor antagonists
- PMID: 31811871
- PMCID: PMC6957304
- DOI: 10.1016/j.neuro.2019.12.001
Susceptibility of larval zebrafish to the seizurogenic activity of GABA type A receptor antagonists
Abstract
Previous studies demonstrated that pentylenetetrazole (PTZ), a GABA type A receptor (GABAAR) antagonist, elicits seizure-like phenotypes in larval zebrafish (Danio rerio). Here, we determined whether the GABAAR antagonists, tetramethylenedisulfotetramine (TETS) and picrotoxin (PTX), both listed as credible chemical threat agents, similarly trigger seizures in zebrafish larvae. Larvae of three, routinely used laboratory zebrafish lines, Tropical 5D, NHGRI and Tupfel long fin, were exposed to varying concentrations of PTZ (used as a positive control), PTX or TETS for 20 min at 5 days post fertilization (dpf). Acute exposure to PTZ, PTX or TETS triggered seizure behavior in the absence of morbidity or mortality. While the concentration-effect relationship for seizure behavior was similar across zebrafish lines for each GABAAR antagonist, significantly less TETS was required to trigger seizures relative to PTX or PTZ. Recordings of extracellular field potentials in the optic tectum of 5 dpf Tropical 5D zebrafish confirmed that all three GABAAR antagonists elicited extracellular spiking patterns consistent with seizure activity, although the pattern varied between chemicals. Post-exposure treatment with the GABAAR positive allosteric modulators (PAMs), diazepam, midazolam or allopregnanolone, attenuated seizure behavior and activity but did not completely normalize electrical field recordings in the optic tectum. These data are consistent with observations of seizure responses in mammalian models exposed to these same GABAAR antagonists and PAMs, further validating larval zebrafish as a higher throughput-screening platform for antiseizure therapeutics, and demonstrating its appropriateness for identifying improved countermeasures for TETS and other convulsant chemical threat agents that trigger seizures via GABAAR antagonism.
Keywords: Chemical threat agents; Picrotoxin; Seizures; TETS; Tetramethylenedisulfotetramine; Zebrafish.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflicts of interest
The authors declare not conflicts of interest
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

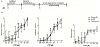
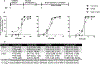

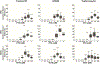




References
-
- Anderson DR, Harris LW, Chang FC, Baze WB, Capacio BR, Byers SL, Lennox WJ. 1997. Antagonism of soman-induced convulsions by midazolam, diazepam and scopolamine. Drug Chem Toxicol. 20(3):115–131. - PubMed
-
- Banks CN, Rogawski MA, Yang D, Lein PJ. 2014. Tetramethylenedisulfotetramine In: Wexler P, editor. Encyclopedia of toxicology. 3rd ed. Oxford, UK: Academic Press; p. 509–511.
-
- Baraban SC, Taylor MR, Castro PA, Baier H. 2005. Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience. 131(3):759–768. - PubMed

