Efficacy of the Enquiring About Tolerance (EAT) study among infants at high risk of developing food allergy
- PMID: 31812184
- PMCID: PMC6902243
- DOI: 10.1016/j.jaci.2019.06.045
Efficacy of the Enquiring About Tolerance (EAT) study among infants at high risk of developing food allergy
Abstract
Background: The Enquiring About Tolerance (EAT) study was a randomized trial of the early introduction of allergenic solids into the infant diet from 3 months of age. The intervention effect did not reach statistical significance in the intention-to-treat analysis of the primary outcome.
Objective: We sought to determine whether infants at high risk of developing a food allergy benefited from early introduction.
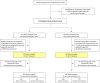
Methods: A secondary intention-to-treat analysis was performed of 3 groups: nonwhite infants; infants with visible eczema at enrollment, with severity determined by SCORAD; and infants with enrollment food sensitization (specific IgE ≥0.1 kU/L).
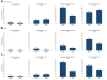
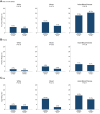
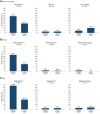
Results: Among infants with sensitization to 1 or more foods at enrollment (≥0.1 kU/L), early introduction group (EIG) infants developed significantly less food allergy to 1 or more foods than standard introduction group (SIG) infants (SIG, 34.2%; EIG, 19.2%; P = .03), and among infants with sensitization to egg at enrollment, EIG infants developed less egg allergy (SIG, 48.6%; EIG, 20.0%; P = .01). Similarly, among infants with moderate SCORAD (15-<40) at enrollment, EIG infants developed significantly less food allergy to 1 or more foods (SIG, 46.7%; EIG, 22.6%; P = .048) and less egg allergy (SIG, 43.3%; EIG, 16.1%; P = .02).
Conclusion: Early introduction was effective in preventing the development of food allergy in specific groups of infants at high risk of developing food allergy: those sensitized to egg or to any food at enrollment and those with eczema of increasing severity at enrollment. This efficacy occurred despite low adherence to the early introduction regimen. This has significant implications for the new national infant feeding recommendations that are emerging around the world.
Keywords: Food allergy; adherence; allergens; breastfeeding; diet; infancy; randomized controlled trial.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
Reply.J Allergy Clin Immunol. 2020 Apr;145(4):1305-1306. doi: 10.1016/j.jaci.2020.01.015. Epub 2020 Feb 25. J Allergy Clin Immunol. 2020. PMID: 32111420 No abstract available.
-
Low-risk infants may still benefit from allergenic food consumption.J Allergy Clin Immunol. 2020 Apr;145(4):1305. doi: 10.1016/j.jaci.2020.01.016. Epub 2020 Feb 25. J Allergy Clin Immunol. 2020. PMID: 32111421 No abstract available.
References
-
- Perkin M.R., Logan K., Tseng A., Raji B., Ayis S., Peacock J. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733–1743. - PubMed
-
- Panjari M., Koplin J.J., Dharmage S.C., Peters R.L., Gurrin L.C., Sawyer S.M. Nut allergy prevalence and differences between Asian-born children and Australian-born children of Asian descent: a state-wide survey of children at primary school entry in Victoria, Australia. Clin Exp Allergy. 2016;46:602–609. - PubMed
-
- Tsakok T., Marrs T., Mohsin M., Baron S., Du T.G., Till S. Does atopic dermatitis cause food allergy? A systematic review. J Allergy Clin Immunol. 2016;137:1071–1078. - PubMed

