Prospective Cohort Study of Childhood-Onset Stargardt Disease: Fundus Autofluorescence Imaging, Progression, Comparison with Adult-Onset Disease, and Disease Symmetry
- PMID: 31812472
- PMCID: PMC7082771
- DOI: 10.1016/j.ajo.2019.11.008
Prospective Cohort Study of Childhood-Onset Stargardt Disease: Fundus Autofluorescence Imaging, Progression, Comparison with Adult-Onset Disease, and Disease Symmetry
Abstract
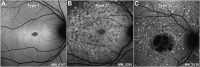
Purpose: To determine the reliability and repeatability of quantitative evaluation of areas of decreased autofluorescence (DAF) from fundus autofluorescence (FAF) images and track disease progression in children with Stargardt disease (STGD1), and to investigate clinical and genotype correlations, disease symmetry, and intrafamilial variability.
Design: Prospective cohort study.
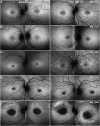
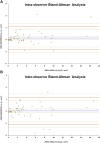
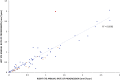
Methods: Children and adults with molecularly confirmed STGD1 (n = 90) underwent longitudinal FAF imaging with subsequent semiautomated measurement of the area of DAF and calculation of the annual rate of progression. The age of disease onset was recorded for all subjects, as well as the electroretinography (ERG) group at baseline (n = 86). Patients were grouped for analysis based on the age at baseline and age of onset, into children (n = 56), adults with childhood-onset STGD1 (n = 15), and adults with adult-onset (n = 19). Fifty FAF images were selected randomly and analyzed by 2 observers to evaluate repeatability and reproducibility. Differences between groups, interocular symmetry, genotype-phenotype correlations, and intrafamilial variability were also investigated both for baseline measurements as well as progression rates. We measured visual acuity, molecular genetics, ERG group, FAF metrics, and their correlations.
Results: The mean age of onset ± SD was 9.6 ± 3.4 years for childhood-onset (n = 71) and 28.3 ± 7.8 years for adult-onset STGD1 (n = 19). The intra- and interobserver reliability of DAF quantification was excellent (intraclass correlation coefficients 0.995 and 0.987, respectively). DAF area was symmetric between eyes and the mean rate of progression (SD) was 0.69 (0.72), 0.78 (0.48), and 0.40 (0.36) mm2/year for children, adults with childhood-onset, and adults with adult-onset disease, respectively. Patients belonging to a group 3 ERG phenotype (generalized cone and rod dysfunction) had a significantly greater progression rate. Limited intrafamilial variability was observed.
Conclusions: This is the first large prospective study of FAF in a cohort of molecularly confirmed children with STGD1. DAF area quantification was highly reliable and may thereby serve as a robust structural endpoint. A high rate of progression was observed in childhood-onset disease, making this subtype of STGD1 ideally suited to be considered for prioritization in clinical trials.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Fujinami K., Lois N., Davidson A.E. A longitudinal study of stargardt disease: clinical and electrophysiologic assessment, progression, and genotype correlations. Am J Ophthalmol. 2013;155(6):1075–1088.e13. - PubMed
-
- Strauss R.W., Ho A., Munoz B. The natural history of the progression of atrophy secondary to Stargardt disease (ProgStar) studies: design and baseline characteristics: ProgStar Report No. 1. Ophthalmology. 2016;123(4):817–828. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

