Development and preclinical pharmacology of a novel dCK inhibitor, DI-87
- PMID: 31812677
- PMCID: PMC6981055
- DOI: 10.1016/j.bcp.2019.113742
Development and preclinical pharmacology of a novel dCK inhibitor, DI-87
Abstract
Background: Deoxycytidine kinase (dCK) is an essential enzyme for production of nucleotides via the salvage pathway; DI-87 is a novel dCK inhibitor in preclinical development for use in anticancer therapy. The current study utilizes PET imaging to evaluate PK-PD relationships and to determine optimal dosing of the drug.
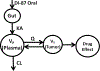
Methods: NSG mice bearing CEM tumors had plasma and tumor PK assessed using mass spectrometry following oral administration of DI-87. dCK inhibition was assessed after a single dose of oral DI-87 followed by a [18F]CFA PET probe and PET imaging. Tumor growth inhibition was assessed by orally administering DI-87 with concurrent intraperitoneal thymidine.
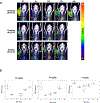
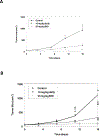
Results: DI-87 had an in vitro EC50 of 10.2 nM with low protein binding. Peak DI-87 concentrations were observed between 1-3 h and 3-9 h in plasma and tumor, respectively, with tumor concentrations less than one third of plasma. Full dCK inhibition, as evaluated by PET imaging, was observed as early as 3 h following 25 mg/kg dosing and was maintained for 12 h, with full recovery of enzyme activity after 36 h. When DI-87 was administered as repeated doses in combination with thymidine, full dCK inhibition was maintained at 12 h (25 mg/kg twice daily dose) and led to maximal tumor growth inhibition.
Conclusions: DI-87 is a promising new compound for use in combination therapy against tumors expressing dCK. Utilizing a [18F]CFA PET probe targeting the pathway of interest allowed for efficient and accurate identification of the optimal dose for growth inhibition.
Keywords: DI-87; Deoxycytidine kinase; PET scan; Pharmacodynamics; Pharmacokinetics; Preclinical.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest:
EVC serves on the data safety and monitoring board for Melinta Pharmaceuticals, Cempra Pharmaceuticals, and The Medicines Company. CGR is a co-inventor of the [18F]CFA probes used in this study. This intellectual property has been patented by the University of California and licensed to Sofie Biosciences, a company that both CGR and the University of California own equity in. In addition, CGR and MEJ are co-inventors of the dCK inhibitors used in this study. This intellectual property has been patented by the University of California and optioned to Trethera Corporation, a company that CGR and MEJ own equity in.
Figures

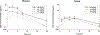

References
-
- Reichard P: Interactions between deoxyribonucleotide and DNA synthesis. Annu Rev Biochem 57:349–74, 1988 - PubMed
-
- Chao J, Synold TW, Morgan RJ Jr., Kunos C, Longmate J, Lenz HJ, et al.: A phase I and pharmacokinetic study of oral 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, NSC #663249) in the treatment of advanced-stage solid cancers: a California Cancer Consortium Study. Cancer Chemother Pharmacol 69:835–43, 2012 - PMC - PubMed

