Computational Markers of Risky Decision-making for Identification of Temporal Windows of Vulnerability to Opioid Use in a Real-world Clinical Setting
- PMID: 31812982
- PMCID: PMC6902203
- DOI: 10.1001/jamapsychiatry.2019.4013
Computational Markers of Risky Decision-making for Identification of Temporal Windows of Vulnerability to Opioid Use in a Real-world Clinical Setting
Abstract
Importance: Opioid addiction is a major public health problem. Despite availability of evidence-based treatments, relapse and dropout are common outcomes. Efforts aimed at identifying reuse risk and gaining more precise understanding of the mechanisms conferring reuse vulnerability are needed.
Objective: To use tools from computational psychiatry and decision neuroscience to identify changes in decision-making processes preceding opioid reuse.
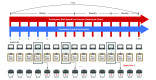
Design, setting, and participants: A cohort of individuals with opioid use disorder were studied longitudinally at a community-based treatment setting for up to 7 months (1-15 sessions per person). At each session, patients completed a risky decision-making task amenable to computational modeling and standard clinical assessments. Time-lagged mixed-effects logistic regression analyses were used to assess the likelihood of opioid use between sessions (t to t + 1; within the subsequent 1-4 weeks) from data acquired at the current session (t). A cohort of control participants completed similar procedures (1-5 sessions per person), serving both as a baseline comparison group and an independent sample in which to assess measurement test-retest reliability. Data were analyzed between January 1, 2018, and September 5, 2019.
Main outcomes and measures: Two individual model-based behavioral markers were derived from the task completed at each session, capturing a participant's current tolerance of known risks and ambiguity (partially unknown risks). Current anxiety, craving, withdrawal, and nonadherence were assessed via interview and clinic records. Opioid use was ascertained from random urine toxicology tests and self-reports.
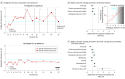
Results: Seventy patients (mean [SE] age, 44.7 [1.3] years; 12 women and 58 men [82.9% male]) and 55 control participants (mean [SE] age, 42.4 [1.5] years; 13 women and 42 men [76.4% male]) were included. Of the 552 sessions completed with patients (mean [SE], 7.89 [0.59] sessions per person), 252 (45.7%) directly preceded opioid use events (mean [SE], 3.60 [0.44] sessions per person). From the task parameters, only ambiguity tolerance was significantly associated with increased odds of prospective opioid use (adjusted odds ratio, 1.37 [95% CI, 1.07-1.76]), indicating patients were more tolerant specifically of ambiguous risks prior to these use events. The association of ambiguity tolerance with prospective use was independent of established clinical factors (adjusted odds ratio, 1.29 [95% CI, 1.01-1.65]; P = .04), such that a model combining these factors explained more variance in reuse risk. No significant differences in ambiguity tolerance were observed between patients and control participants, who completed 197 sessions (mean [SE], 3.58 [0.21] sessions per person); however, patients were more tolerant of known risks (B = 0.56 [95% CI, 0.05-1.07]).
Conclusions and relevance: Computational approaches can provide mechanistic insights about the cognitive factors underlying opioid reuse vulnerability and may hold promise for clinical use.
Conflict of interest statement
Figures

References
-
- Kerlikowske RG. National drug control strategy: data supplement. https://obamawhitehouse.archives.gov/sites/default/files/ondcp/policy-an.... Published 2013. Accessed November 25, 2019.
-
- National Institute on Drug Abuse Trends & statistics. https://www.drugabuse.gov/related-topics/trends-statistics#costs. Published April 2017. Accessed November 26, 2019.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

