Effect of Additional Rehabilitation After Botulinum Toxin-A on Upper Limb Activity in Chronic Stroke: The InTENSE Trial
- PMID: 31813359
- PMCID: PMC7004444
- DOI: 10.1161/STROKEAHA.119.027602
Effect of Additional Rehabilitation After Botulinum Toxin-A on Upper Limb Activity in Chronic Stroke: The InTENSE Trial
Erratum in
-
Correction to: Effect of Additional Rehabilitation After Botulinum Toxin-A on Upper Limb Activity in Chronic Stroke: The InTENSE Trial.Stroke. 2020 Feb;51(2):e45. doi: 10.1161/STR.0000000000000219. Epub 2020 Jan 27. Stroke. 2020. PMID: 31986111 Free PMC article. No abstract available.
Abstract
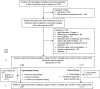
Background and Purpose- The aim of this trial was to determine the effect of additional upper limb rehabilitation following botulinum toxin-A for upper limb activity in chronic stroke. Methods- We conducted a multicenter phase III randomized trial with concealed allocation, blinded measurement, and intention-to-treat analysis. One hundred forty stroke survivors who were scheduled to receive botulinum toxin-A in any muscle(s) that cross the wrist because of moderate to severe spasticity after a stroke >3 months ago, who had completed formal rehabilitation and had no significant cognitive impairment. Experimental group received botulinum toxin-A plus evidence-based movement training while the control group received botulinum toxin-A plus a handout of exercises. Primary outcomes were goal attainment (Goal Attainment Scaling) and upper limb activity (Box and Block Test) at 3 months (end of intervention). Secondary outcomes were spasticity, range of motion, strength, pain, burden of care, and health-related quality of life. Results- In terms of goal attainment, the experimental group scored the same (mean difference, 2 T-score [95% CI, -2 to 7]) as the control group on the Goal Attainment Scale. In terms of upper limb activity, by 3 months the experimental group moved blocks at the same speed (mean difference, 0.00 blocks/s [95% CI, -0.02 to 0.01]) as the control group on the Box and Block Test. There were no differences between groups on any secondary outcome except strength, in favor of the experimental group (mean difference, 1.4 kg [95% CI, 0.2-2.7]). Conclusions- Findings suggest that additional intensive upper limb rehabilitation following botulinum toxin-A in chronic stroke survivors with a disabled upper limb is not effective. Registration- URL: https://www.clinicaltrials.gov. Unique identifier: ACTRN12615000616572.
Keywords: botulinum toxin type A; neuroscience; pain; quality of life; spasticity; wrist.
Figures
Comment in
-
Critically appraised paper: Additional rehabilitation following botulinum toxin-A does not improve goal attainment and upper limb activity in chronic stroke survivors [synopsis].J Physiother. 2021 Jul;67(3):217. doi: 10.1016/j.jphys.2021.05.005. Epub 2021 May 28. J Physiother. 2021. PMID: 34053900 No abstract available.
References
-
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2019 update: a Report From the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. - PubMed
-
- Cardoso E, Rodrigues B, Lucena R, Oliveira IR, Pedreira G, Melo A. Botulinum toxin type A for the treatment of the upper limb spasticity after stroke: a meta-analysis. Arq Neuropsiquiatr. 2005;63:30–33. doi: 10.1590/s0004-282x2005000100006. - PubMed
-
- Mills PB, Finlayson H, Sudol M, O’Connor R. Systematic review of adjunct therapies to improve outcomes following botulinum toxin injection for treatment of limb spasticity. Clin Rehabil. 2016;30:537–548. doi: 10.1177/0269215515593783. - PubMed
-
- Kinnear BZ, Lannin NA, Cusick A, Harvey LA, Rawicki B. Rehabilitation therapies after botulinum toxin-A injection to manage limb spasticity: a systematic review. Phys Ther. 2014;94:1569–1581. doi: 10.2522/ptj.20130408. - PubMed
-
- Sheean G, Lannin NA, Turner-Stokes L, Rawicki B, Snow BJ Cerebral Palsy Institute. Botulinum toxin assessment, intervention and after-care for upper limb hypertonicity in adults: international consensus statement. Eur J Neurol. 2010;17(suppl 2):74–93. doi: 10.1111/j.1468-1331.2010.03129.x. - PubMed