Activity-Dependent Nucleation of Dynamic Microtubules at Presynaptic Boutons Controls Neurotransmission
- PMID: 31813605
- PMCID: PMC6917861
- DOI: 10.1016/j.cub.2019.10.049
Activity-Dependent Nucleation of Dynamic Microtubules at Presynaptic Boutons Controls Neurotransmission
Abstract
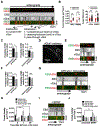
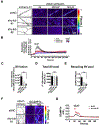
Control of microtubule (MT) nucleation and dynamics is critical for neuronal function. Whether MT nucleation is regulated at presynaptic boutons and influences overall presynaptic activity remains unknown. By visualizing MT plus-end dynamics at individual excitatory en passant boutons in axons of cultured hippocampal neurons and in hippocampal slices expressing EB3-EGFP and vGlut1-mCherry, we found that dynamic MTs preferentially grow from presynaptic boutons, show biased directionality in that they are almost always oriented toward the distal tip of the axon, and can be induced by neuronal activity. Silencing of γ-tubulin expression reduced presynaptic MT nucleation, and depletion of either HAUS1 or HAUS7-augmin subunits increased the percentage of retrograde comets initiated at boutons, indicating that γ-tubulin and augmin are required for activity-dependent de novo nucleation of uniformly distally oriented dynamic MTs. We analyzed the dynamics of a wide range of axonal organelles as well as synaptic vesicles (SVs) relative to vGlut1+ stable presynaptic boutons in a time window during which MT nucleation at boutons is promoted upon induction of neuronal activity, and we found that γ-tubulin-dependent presynaptic MT nucleation controls bidirectional (SV) interbouton transport and regulates evoked SV exocytosis. Hence, en passant boutons act as hotspots for activity-dependent de novo MT nucleation, which controls neurotransmission by providing dynamic tracks for bidirectional delivery of SVs between sites of neurotransmitter release.
Keywords: augmin; dynamic microtubules; microtubule nucleation; presynaptic boutons; synaptic vesicles; γ-tubulin.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
Figures


Comment in
-
Neuronal Cytoskeleton: Presynaptic Boutons as Hotspots for Activity-Dependent Microtubule Nucleation.Curr Biol. 2019 Dec 16;29(24):R1307-R1309. doi: 10.1016/j.cub.2019.11.011. Curr Biol. 2019. PMID: 31846677
References
-
- Kapitein LC, and Hoogenraad CC (2015). Building the Neuronal Microtubule Cytoskeleton. Neuron 87, 492–506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

