T Cell Antifungal Immunity and the Role of C-Type Lectin Receptors
- PMID: 31813764
- PMCID: PMC7427322
- DOI: 10.1016/j.it.2019.11.007
T Cell Antifungal Immunity and the Role of C-Type Lectin Receptors
Abstract
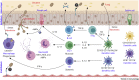
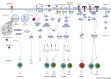
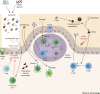
Fungi can cause disease in humans, from mucocutaneous to life-threatening systemic infections. Initiation of antifungal immunity involves fungal recognition by pattern recognition receptors such as C-type lectin receptors (CLRs). These germline-encoded receptors trigger a multitude of innate responses including phagocytosis, fungal killing, and antigen presentation which can also shape the development of adaptive immunity. Recently, studies have shed light on how CLRs directly or indirectly modulate lymphocyte function. Moreover, CLR-mediated recognition of commensal fungi maintains homeostasis and prevents invasion from opportunistic commensals. We present an overview of current knowledge of antifungal T cell immune responses, with emphasis on the role of C-type lectins, and discuss how these receptors modulate these responses at different levels.
Keywords: C-type lectin receptors; adaptive T cell immunity; antifungal immunity; fungal pathogens; mycobiome.
Copyright © 2019 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Feigal D.W. The prevalence of oral lesions in HIV-infected homosexual and bisexual men: three San Francisco epidemiological cohorts. AIDS. 1991;5:519–525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

