Regulation of Tumor Initiation by the Mitochondrial Pyruvate Carrier
- PMID: 31813825
- PMCID: PMC7004878
- DOI: 10.1016/j.cmet.2019.11.002
Regulation of Tumor Initiation by the Mitochondrial Pyruvate Carrier
Abstract
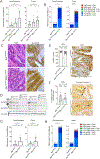
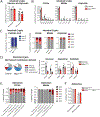
Although metabolic adaptations have been demonstrated to be essential for tumor cell proliferation, the metabolic underpinnings of tumor initiation are poorly understood. We found that the earliest stages of colorectal cancer (CRC) initiation are marked by a glycolytic metabolic signature, including downregulation of the mitochondrial pyruvate carrier (MPC), which couples glycolysis and glucose oxidation through mitochondrial pyruvate import. Genetic studies in Drosophila suggest that this downregulation is required because hyperplasia caused by loss of the Apc or Notch tumor suppressors in intestinal stem cells can be completely blocked by MPC overexpression. Moreover, in two distinct CRC mouse models, loss of Mpc1 prior to a tumorigenic stimulus doubled the frequency of adenoma formation and produced higher grade tumors. MPC loss was associated with a glycolytic metabolic phenotype and increased expression of stem cell markers. These data suggest that changes in cellular pyruvate metabolism are necessary and sufficient to promote cancer initiation.
Keywords: cancer metabolism; carbohydrate metabolism; colon cancer; mitochondria; pyruvate metabolism; stem cell metabolism; tumor initiation.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The University of Utah has filed a patent related to the mitochondrial pyruvate carrier, of which J.R. and C.S.T. are listed as co-inventors. All other authors declare no competing interests.
Figures





References
-
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, and Clevers H (2009). Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457, 608–611. - PubMed
-
- Berg JA. (2019). j-berg/bensard_figures_2019: Manuscript Resubmission (update) (Version v0.0.2). Zenodo. 10.5281/zenodo.3379036 - DOI
-
- Berg JA, Belyeu JR, Morgan JT, Bott AJ, Ouyang Y, Quinlan AR, Gertz J, Rutter J (2019). XPRESSyourself: Enhancing and Automating the Ribosome Profiling and RNA-Seq Analysis Toolkit. bioRxiv 704320; doi: 10.1101/704320 - DOI
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM122778/GM/NIGMS NIH HHS/United States
- F30 CA225110/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- P40 OD018537/OD/NIH HHS/United States
- K00 CA212445/CA/NCI NIH HHS/United States
- T32 DK091317/DK/NIDDK NIH HHS/United States
- T32 HL007576/HL/NHLBI NIH HHS/United States
- T32 DK110966/DK/NIDDK NIH HHS/United States
- S10 OD016232/OD/NIH HHS/United States
- S10 OD018210/OD/NIH HHS/United States
- R00 CA215307/CA/NCI NIH HHS/United States
- U54 DK110858/DK/NIDDK NIH HHS/United States
- T32 HD007491/HD/NICHD NIH HHS/United States
- S10 OD021505/OD/NIH HHS/United States
- R01 CA228346/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

