G2019S-LRRK2 mutation enhances MPTP-linked Parkinsonism in mice
- PMID: 31813996
- PMCID: PMC7068031
- DOI: 10.1093/hmg/ddz271
G2019S-LRRK2 mutation enhances MPTP-linked Parkinsonism in mice
Abstract
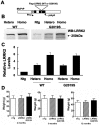
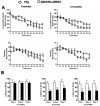
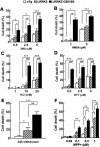
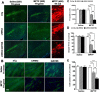
Parkinson's disease (PD) is a common neurodegenerative disease with a heterogeneous etiology that involves genetic and environmental factors or exogenous. Current LRRK2 PD animal models only partly reproduce the characteristics of the disease with very subtle dopaminergic neuron degeneration. We developed a new model of PD that combines a sub-toxic MPTP insult to the G2019S-LRRK2 mutation. Our newly generated mice, overexpressing mutant G2019S-LRRK2 protein in the brain, displayed a mild, age-dependent progressive motor impairment, but no reduction of lifespan. Cortical neurons from G2019S-LRRK2 mice showed an increased vulnerability to stress insults, compared with neurons overexpressing wild-type WT-LRRK2, or non-transgenic (nTg) neurons. The exposure of LRRK2 transgenic mice to a sub-toxic dose of MPTP resulted in severe motor impairment, selective loss of dopamine neurons and increased astrocyte activation, whereas nTg mice with MPTP exposure showed no deficits. Interestingly, mice overexpressing WT-LRRK2 showed a significant impairment that was milder than for the mutant G2019S-LRRK2 mice. L-DOPA treatments could partially improve the movement impairments but did not protect the dopamine neuron loss. In contrast, treatments with an LRRK2 kinase inhibitor significantly reduced the dopaminergic neuron degeneration in this interaction model. Our studies provide a novel LRRK2 gene-MPTP interaction PD mouse model, and a useful tool for future studies of PD pathogenesis and therapeutic intervention.
© The Author(s) 2019. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Tysnes O.B. and Storstein A. (2017) Epidemiology of Parkinson's disease. J. Neural Transm., 124, 901–905. - PubMed
-
- Poewe W. (2017) Parkinson's disease and the quest for preclinical diagnosis: an interview with Professor Werner Poewe. Neurodegener Dis. Manag., 7, 273–277. - PubMed
-
- Deng H., Wang P. and Jankovic J. (2018) The genetics of Parkinson disease. Ageing Res. Rev., 42, 72–85. - PubMed
-
- Smith W.W., Pei Z., Jiang H., Dawson V.L., Dawson T.M. and Ross C.A. (2006) Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat. Neurosci., 9, 1231–1233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

