Durvalumab for the Treatment of Locally Advanced, Unresectable, Stage III Non-Small Cell Lung Cancer: An Evidence Review Group Perspective of a NICE Single Technology Appraisal
- PMID: 31814080
- PMCID: PMC7080309
- DOI: 10.1007/s40273-019-00870-w
Durvalumab for the Treatment of Locally Advanced, Unresectable, Stage III Non-Small Cell Lung Cancer: An Evidence Review Group Perspective of a NICE Single Technology Appraisal
Abstract
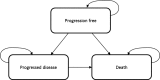
As part of the Single Technology Appraisal (STA) process, the National Institute for Health and Care Excellence (NICE) invited the manufacturer (AstraZeneca) of durvalumab (IMFINZITM) to submit evidence for the clinical and cost effectiveness of durvalumab for the treatment of patients with locally advanced, unresectable, stage III non-small cell lung cancer whose tumours express programmed death-ligand 1 (PD-L1) on ≥ 1% of tumour cells and whose disease has not progressed after platinum-based chemoradiation therapy. Kleijnen Systematic Reviews Ltd, in collaboration with Maastricht University Medical Centre, was commissioned to act as the independent Evidence Review Group (ERG). This paper summarises the company submission (CS), presents the ERG's critical review on the clinical- and cost-effectiveness evidence in the CS, highlights the key methodological considerations, and describes the development of the NICE guidance by the Appraisal Committee. The CS included a systematic review that identified one randomised controlled trial, comparing durvalumab with SoC. Participants with tumours expressing PD-L1 on ≥ 1% of tumour cells accounted for approximately 40% of the total participants. In this subgroup, a benefit in progression-free survival (PFS) [hazard ratio (HR) 0.44, 95% confidence interval (CI) 0.31-0.63] and overall survival (HR 0.54, 95% CI 0.35-0.81) was reported. Adverse events were comparable between both treatments, but more serious adverse events were reported for durvalumab (64/213 [30%] vs. 18/90 [20%]). The ERG's concerns regarding the economic analysis included a likely overestimation of PFS for the durvalumab arm, the choice of timepoint for treatment waning, as well as the way treatment waning was incorporated in the model, and potential overestimation of utility values without applying an age- or treatment-related decrement. The revised ERG base-case resulted in a deterministic incremental cost-effectiveness ratio of £50,238 per quality-adjusted life-year gained, with substantial remaining uncertainty. NICE recommended durvalumab as an option for use within the Cancer Drugs Fund only in a subpopulation (concurrent platinum-based chemoradiation therapy) with a commercially managed access agreement in place.
Conflict of interest statement
Willem Witlox, Antoinette van Asselt, Robert Wolff, Nigel Armstrong, Gill Worthy, Annette Chalker, Titas Buksnys, Lisa Stirk, Jos Kleijnen, Manuela Joore and Sabine Grimm have no conflicts of interest to declare.
Figures
References
-
- National Institute for Health and Care Excellence. Durvalumab for treating locally advanced unresectable non-small-cell lung cancer after platinum-based chemoradiation [TA578]. 2019. https://www.nice.org.uk/guidance/ta578/evidence. Accessed 22 Aug 2019.
-
- AstraZeneca. Durvalumab for treatment of locally advanced, unresectable, stage III non-small cell lung cancer in adults whose tumours express PD-L1 on >=1% of tumour cells and whose disease has not progressed after platinum-based chemo-radiation therapy (ID1175): Submission to National Institute of Health and Care Excellence. Single technology appraisal (STA): Document B: Appendices. AstraZeneca; 2018. pp. 356.
-
- AstraZeneca. A phase III, randomized, double-blind, placebo-controlled, multi-center, international study of MEDI4736 as sequential therapy in patients with locally advanced, unresectable non-small cell lung cancer (stage III) who have not progressed following definitive, platinum-based, concurrent chemoradiation therapy (PACIFIC) [Clinical Study Report: durvalumab]. 21 July 2017. Data on File. (PDF provided by the company).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials