Polyphyllin VII Promotes Apoptosis and Autophagic Cell Death via ROS-Inhibited AKT Activity, and Sensitizes Glioma Cells to Temozolomide
- PMID: 31814867
- PMCID: PMC6877958
- DOI: 10.1155/2019/1805635
Polyphyllin VII Promotes Apoptosis and Autophagic Cell Death via ROS-Inhibited AKT Activity, and Sensitizes Glioma Cells to Temozolomide
Abstract
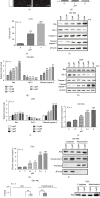
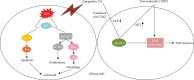
The high recurrence frequency of gliomas but deficiency of effective treatment and prevalent chemoresistance have elicited interests in exploring and developing new agents. Paris polyphyllins are monomers extracted from rhizome of Paris polyphylla var. yunnanensis. Here, we first reported that polyphyllin VII (PP7) exhibited cytotoxic effect on glioma cells. PP7 significantly suppressed the viability and induced cell death of U87-MG and U251 cells after 24 h, with the IC50 values 4.24 ± 0.87 μM and 2.17 ± 0.14 μM, respectively. Both apoptotic and autophagic processes were involved in the cytotoxic effect of PP7, as PP7 activated the Bcl2/Bax pathway and the inhibition of autophagy partly rescued the toxicity of PP7 in glioma cells. In addition, an inhibition of AKT/mTORC1 activity was found after PP7 administration, and it seemed that the overproduction of reactive oxygen species (ROS) was responsible for this effect. Namely, the removal of ROS by NAC treatment mitigated PP7-induced cell death, autophagy, and its effect on the AKT/mTORC1 signaling. Additionally, a combination assay of PP7 with temozolomide (TMZ), the most used chemotherapy for glioma patients, was performed resulting in synergism, while PP7 reduced TMZ resistance through inhibition of MGMT expression. Thus, our study reports PP7 as a potential agent for glioma treatment and reveals its underlying mechanisms of action.
Copyright © 2019 Dejiang Pang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures






References
-
- Rock K., McArdle O., Forde P., et al. A clinical review of treatment outcomes in glioblastoma multiforme—the validation in a non-trial population of the results of a randomised phase III clinical trial: has a more radical approach improved survival? The British Journal of Radiology. 2012;85(1017):e729–e733. doi: 10.1259/bjr/83796755. - DOI - PMC - PubMed
-
- Ostrom Q. T., Gittleman H., Truitt G., Boscia A., Kruchko C., Barnholtz-Sloan J. S. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro-Oncology. 2018;20(Supplement 4):iv1–iv86. doi: 10.1093/neuonc/noy131. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

