Astragaloside II alleviates the symptoms of experimental ulcerative colitis in vitro and in vivo
- PMID: 31814910
- PMCID: PMC6895531
Astragaloside II alleviates the symptoms of experimental ulcerative colitis in vitro and in vivo
Abstract
Background: Ulcerative colitis (UC) is a chronic inflammatory intestinal disease, and its morbidity is rising worldwide. Previous study indicated that astragaloside II (AS II), a monomeric compound, was used to treat bowel disease. However, the effects of AS II on UC remains unclear. Thus, this study aimed to investigate the therapeutic effects of AS II on experimental UC in vitro and in vivo.
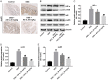
Methods: CCD-18Co cells were stimulated by 1 μg/mL LPS to mimic UC in vitro. In addition, dextran sulfate sodium (DSS)-induced UC mouse model was established in vivo. CCK-8 assay was used to detect cell proliferation in vitro. Moreover, the concentrations of inflammatory factors interleukin 6 (IL-6), tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β), nitric oxide (NO), superoxide dismutase (SOD) and malondialdehyde (MDA) in CCD-18Co cells and colon tissues were determined by ELISA, respectively. Meanwhile, the expressions of hypoxia-inducible factor 1α (HIF-α), phospho-inhibitor of NF-κB (p-IκB) and phospho-NF-κB p65 (p-p65) were detected by western blotting in vitro and in vivo, respectively.
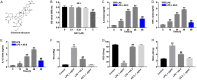
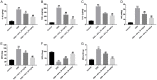
Results: In this study, the levels of pro-inflammatory cytokines TNF-α, IL-1β and IL-6 were significantly increased in lipopolysaccharide (LPS)-stimulated CCD-18Co cells. However, LPS-induced inflammatory response was markedly alleviated by AS II. In addition, LPS-induced HIF-α, p-IκB and p-p65 proteins increases were markedly ameliorated by AS II treatment. Moreover, AS II reduced disease activity index (DAI) scores and increased the colon lengths in DSS-treated mice. Meanwhile, AS II decreased the levels of IL-6, TNF-α, IL-1β, NO, MPO and MDA, and increased the level of SOD in colon of DSS-treated mice. Furthermore, AS II downregulated the expressions of HIF-α, p-IκB and p-p65 in DSS-induced UC in mice.
Conclusion: Our findings indicated that AS II could alleviate inflammatory response in LPS-induced CCD-18Co cells and in DSS-induced UC in mice. In conclusion, AS II may serve as a potential agent for the treatment of UC.
Keywords: Astragaloside II; dextran sulfate sodium; lipopolysaccharide; ulcerative colitis.
AJTR Copyright © 2019.
Conflict of interest statement
None.
Figures


Similar articles
-
Astragaloside IV alleviates the symptoms of experimental ulcerative colitis in vitro and in vivo.Exp Ther Med. 2019 Oct;18(4):2877-2884. doi: 10.3892/etm.2019.7907. Epub 2019 Aug 16. Exp Ther Med. 2019. PMID: 31572532 Free PMC article.
-
The in vitro and in vivo anti-inflammatory effect of osthole, the major natural coumarin from Cnidium monnieri (L.) Cuss, via the blocking of the activation of the NF-κB and MAPK/p38 pathways.Phytomedicine. 2019 May;58:152864. doi: 10.1016/j.phymed.2019.152864. Epub 2019 Feb 18. Phytomedicine. 2019. PMID: 30878874
-
Anti-inflammatory effects of Brucea javanica oil emulsion by suppressing NF-κB activation on dextran sulfate sodium-induced ulcerative colitis in mice.J Ethnopharmacol. 2017 Feb 23;198:389-398. doi: 10.1016/j.jep.2017.01.042. Epub 2017 Jan 22. J Ethnopharmacol. 2017. PMID: 28119098
-
Baicalin may alleviate inflammatory infiltration in dextran sodium sulfate-induced chronic ulcerative colitis via inhibiting IL-33 expression.Life Sci. 2017 Oct 1;186:125-132. doi: 10.1016/j.lfs.2017.08.010. Epub 2017 Aug 9. Life Sci. 2017. PMID: 28802904
-
Pharmacological effects of berberine on models of ulcerative colitis: A meta-analysis and systematic review of animal studies.Front Pharmacol. 2022 Sep 6;13:937029. doi: 10.3389/fphar.2022.937029. eCollection 2022. Front Pharmacol. 2022. PMID: 36147325 Free PMC article.
Cited by
-
Astragaloside II Ameliorated Podocyte Injury and Mitochondrial Dysfunction in Streptozotocin-Induced Diabetic Rats.Front Pharmacol. 2021 Mar 16;12:638422. doi: 10.3389/fphar.2021.638422. eCollection 2021. Front Pharmacol. 2021. PMID: 33796024 Free PMC article.
-
Identification strategy of wild and cultivated Astragali Radix based on REIMS combined with two-dimensional LC-MS.NPJ Sci Food. 2024 Nov 8;8(1):91. doi: 10.1038/s41538-024-00333-3. NPJ Sci Food. 2024. PMID: 39516475 Free PMC article.
-
Chinese botanical drugs targeting mitophagy to alleviate diabetic kidney disease, a comprehensive review.Front Pharmacol. 2024 May 13;15:1360179. doi: 10.3389/fphar.2024.1360179. eCollection 2024. Front Pharmacol. 2024. PMID: 38803440 Free PMC article. Review.
-
Protective Effect and Related Mechanism of Modified Danggui Buxue Decoction on Retinal Oxidative Damage in Mice based on Network Pharmacology.Curr Pharm Des. 2024;30(24):1912-1926. doi: 10.2174/0113816128293824240517113238. Curr Pharm Des. 2024. PMID: 38835123
-
Identification of anti-inflammatory components in Panax ginseng of Sijunzi Decoction based on spectrum-effect relationship.Chin Herb Med. 2022 Oct 17;15(1):123-131. doi: 10.1016/j.chmed.2022.04.003. eCollection 2023 Jan. Chin Herb Med. 2022. PMID: 36875431 Free PMC article.
References
-
- Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology. 2017;152:313–321. e2. - PubMed
-
- Hata K, Kishikawa J, Anzai H, Shinagawa T, Kazama S, Ishii H, Nozawa H, Kawai K, Kiyomatsu T, Tanaka J, Tanaka T, Nishikawa T, Otani K, Yasuda K, Yamaguchi H, Ishihara S, Sunami E, Kitayama J, Watanabe T. Surveillance colonoscopy for colitis-associated dysplasia and cancer in ulcerative colitis patients. Dig Endosc. 2016;28:260–265. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
