Glycans in drug discovery
- PMID: 31814952
- PMCID: PMC6839814
- DOI: 10.1039/c9md00292h
Glycans in drug discovery
Abstract
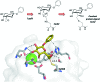
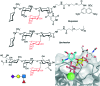
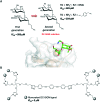
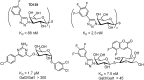
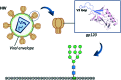
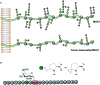
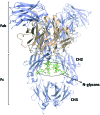
Glycans are key players in many biological processes. They are essential for protein folding and stability and act as recognition elements in cell-cell and cell-matrix interactions. Thus, being at the heart of medically relevant biological processes, glycans have come onto the scene and are considered hot spots for biomedical intervention. The progress in biophysical techniques allowing access to an increasing molecular and structural understanding of these processes has led to the development of effective therapeutics. Indeed, strategies aimed at designing glycomimetics able to block specific lectin-carbohydrate interactions, carbohydrate-based vaccines mimicking self- and non-self-antigens as well as the exploitation of the therapeutic potential of glycosylated antibodies are being pursued. In this mini-review the most prominent contributions concerning recurrent diseases are highlighted, including bacterial and viral infections, cancer or immune-related pathologies, which certainly show the great promise of carbohydrates in drug discovery.
This journal is © The Royal Society of Chemistry 2019.
Figures



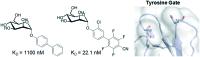

References
-
- Cummings R. D. and Etzler M. E., Essentials of Glycobiology, ed. A. Varki, R. D. Cummings, J. D. Esko, H. H. Freeze, P. Stanley, C. R. Bertozzi, G. W. Hart and M. E. Etzler, Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press, New York, 2nd edn, 2009, ch. 6. - PubMed
-
- Joshi H. J., Narimatsu Y., Schjoldager K. T., Tytgat H. L. P., Aebi M., Clausen H., Halim A. Cell. 2018;172:632–632.e2. - PubMed
-
- Schwarz F., Aebi M. Curr. Opin. Struct. Biol. 2011;21:576–582. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

