Disulfiram combined with copper induces immunosuppression via PD-L1 stabilization in hepatocellular carcinoma
- PMID: 31815045
- PMCID: PMC6895448
Disulfiram combined with copper induces immunosuppression via PD-L1 stabilization in hepatocellular carcinoma
Abstract
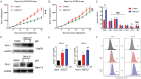
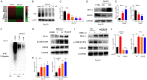
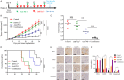
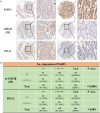
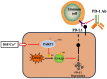
As a potential antitumor drug and chemotherapeutic sensitizer, disulfiram combined with Copper (DSF/Cu2+) does not exert considerable antitumor effects on an immunocompetent hepatocellular carcinoma (HCC) model. In this article, we will explore the mechanism underlying the resistance to DSF in HCC. We analyzed the antitumor effect of DSF/Cu2+ in vivo studies. Tumor and immune cells collected from mice were analyzed by flow cytometry. Then, we analyzed the transcriptional changes in liver cancer cells after DSF/Cu2+ treatment by transcriptional expression profiling. The expression of PD-L1 was detected by real-time PCR, Western blotting and flow cytometry. The expression of PARP1 and GSK3β was knocked down by small interfering RNAs (siRNAs). A subcutaneous Hepa1-6 tumor model was used for single-drug or combined-drug studies. Tissue chips (268 samples of liver cancer tissue) were used to analyze the relationship among PARP1, p-GSK3β and PD-L1. We found that DSF/Cu2+ failed to inhibit HCC tumor growth in C57BL/6 mice. DSF/Cu2+ upregulated PD-L1 expression by inhibiting PARP1 activity and enhancing GSK3β phosphorylation at Ser9 and ultimately inhibited T cell infiltration. The combination of DSF/Cu2+ and an anti-PD-1 antibody produced an additive effect that slowed HCC growth in mice. In addition, we observed negative associations between PARP1 and p-GSK3β (Ser9) or PD-L1 expression in tumor tissue samples from HCC patients. Through in vitro and in vivo studies, we found that DSF/Cu2+ could restrain GSK3β activity by inhibiting PARP1, leading to the upregulation of PD-L1 expression. Combination therapy with DSF/Cu2+ and an anti-PD-1 antibody showed much better antitumor efficacy than monotherapy.
Keywords: GSK3; PARP1; PD-L1; disulfiram; hepatocellular carcinoma.
AJCR Copyright © 2019.
Conflict of interest statement
None.
Figures
References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. - PubMed
-
- Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. - PubMed
-
- Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
