Pembrolizumab microgravity crystallization experimentation
- PMID: 31815178
- PMCID: PMC6889310
- DOI: 10.1038/s41526-019-0090-3
Pembrolizumab microgravity crystallization experimentation
Abstract
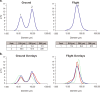
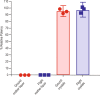
Crystallization processes have been widely used in the pharmaceutical industry for the manufacture, storage, and delivery of small-molecule and small protein therapeutics. However, the identification of crystallization processes for biologics, particularly monoclonal antibodies, has been prohibitive due to the size and the flexibility of their overall structure. There remains a challenge and an opportunity to utilize the benefits of crystallization of biologics. The research laboratories of Merck Sharp & Dome Corp. (MSD) in collaboration with the International Space Station (ISS) National Laboratory performed crystallization experiments with pembrolizumab (Keytruda®) on the SpaceX-Commercial Resupply Services-10 mission to the ISS. By leveraging microgravity effects such as reduced sedimentation and minimal convection currents, conditions producing crystalline suspensions of homogeneous monomodal particle size distribution (39 μm) in high yield were identified. In contrast, the control ground experiments produced crystalline suspensions with a heterogeneous bimodal distribution of 13 and 102 μm particles. In addition, the flight crystalline suspensions were less viscous and sedimented more uniformly than the comparable ground-based crystalline suspensions. These results have been applied to the production of crystalline suspensions on earth, using rotational mixers to reduce sedimentation and temperature gradients to induce and control crystallization. Using these techniques, we have been able to produce uniform crystalline suspensions (1-5 μm) with acceptable viscosity (<12 cP), rheological, and syringeability properties suitable for the preparation of an injectable formulation. The results of these studies may help widen the drug delivery options to improve the safety, adherence, and quality of life for patients and caregivers.
Keywords: Biochemistry; Biophysical chemistry.
© The Author(s) 2019.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures



References
-
- Jensen, L. H. & Stout, G. H. X-ray Structure Determination, A Practical Guide X-Ray Structure Determination: A Practical Guide 2nd edn, Vol. 67 (1) (Reuben Rudman Journal of Chemical Education, 1990).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

