Preserved wake-dependent cortical excitability dynamics predict cognitive fitness beyond age-related brain alterations
- PMID: 31815203
- PMCID: PMC6890637
- DOI: 10.1038/s42003-019-0693-y
Preserved wake-dependent cortical excitability dynamics predict cognitive fitness beyond age-related brain alterations
Erratum in
-
Author Correction: Preserved wake-dependent cortical excitability dynamics predict cognitive fitness beyond age-related brain alterations.Commun Biol. 2021 Apr 8;4(1):472. doi: 10.1038/s42003-021-01995-5. Commun Biol. 2021. PMID: 33833380 Free PMC article. No abstract available.
Abstract
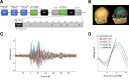
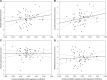
Age-related cognitive decline arises from alterations in brain structure as well as in sleep-wake regulation. Here, we investigated whether preserved wake-dependent regulation of cortical function could represent a positive factor for cognitive fitness in aging. We quantified cortical excitability dynamics during prolonged wakefulness as a sensitive marker of age-related alteration in sleep-wake regulation in 60 healthy older individuals (50-69 y; 42 women). Brain structural integrity was assessed with amyloid-beta- and tau-PET, and with MRI. Participants' cognition was investigated using an extensive neuropsychological task battery. We show that individuals with preserved wake-dependent cortical excitability dynamics exhibit better cognitive performance, particularly in the executive domain which is essential to successful cognitive aging. Critically, this association remained significant after accounting for brain structural integrity measures. Preserved dynamics of basic brain function during wakefulness could therefore be essential to cognitive fitness in aging, independently from age-related brain structural modifications that can ultimately lead to dementia.
Keywords: Cognitive ageing; Wakefulness.
© The Author(s) 2019.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

