Chinese wheat mosaic virus-derived vsiRNA-20 can regulate virus infection in wheat through inhibition of vacuolar- (H+ )-PPase induced cell death
- PMID: 31815302
- PMCID: PMC7065157
- DOI: 10.1111/nph.16358
Chinese wheat mosaic virus-derived vsiRNA-20 can regulate virus infection in wheat through inhibition of vacuolar- (H+ )-PPase induced cell death
Abstract
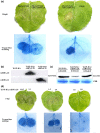
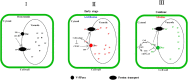
Vacuolar (H+ )-PPases (VPs), are key regulators of active proton (H+ ) transport across membranes using the energy generated from PPi hydrolysis. The VPs also play vital roles in plant responses to various abiotic stresses. Their functions in plant responses to pathogen infections are unknown. Here, we show that TaVP, a VP of wheat (Triticum aestivum) is important for wheat resistance to Chinese wheat mosaic virus (CWMV) infection. Furthermore, overexpression of TaVP in plants induces the activity of PPi hydrolysis, leading to plants cell death. A virus-derived small interfering RNA (vsiRNA-20) generated from CWMV RNA1 can regulate the mRNA accumulation of TaVP in wheat. The accumulation of vsiRNA-20 can suppress cell death induced by TaVP in a dosage-dependent manner. Moreover, we show that the accumulation of vsiRNA-20 can affect PPi hydrolysis and the concentration of H+ in CWMV-infected wheat cells to create a more favorable cellular environment for CWMV replication. We propose that vsiRNA-20 regulates TaVP expression to prevent cell death and to maintain a weak alkaline environment in cytoplasm to enhance CWMV infection in wheat. This finding may be used as a novel strategy to minimize virus pathogenicity and to develop new antiviral stratagems.
Keywords: Chinese wheat mosaic virus (CWMV); pathogenicity; vacuolar (H+)-PPase (VP); virus-derived small interfering RNA (vsiRNA); wheat.
© 2019 The Authors. New Phytologist © 2019 New Phytologist Trust.
Figures








References
-
- Adams MJ, Antoniw JF, Kreuze J. 2009. Virgaviridae: a new family of rod‐shaped plant viruses. Archives of Virology 154: 1967–1972. - PubMed
-
- Andika IB, Zheng S, Tan Z, Sun L, Kondo H, Zhou X, Chen J. 2013. Endoplasmic reticulum export and vesicle formation of the movement protein of Chinese wheat mosaic virus are regulated by two transmembrane domains and depend on the secretory pathway. Virology 435: 493–503. - PubMed
-
- Brini F, Hanin M, Mezghani I, Berkowitz GA, Masmoudi K. 2007. Overexpression of wheat Na+/H+ antiporter TNHX1 and H+‐pyrophosphatase TVP1 improve salt‐ and drought‐stress tolerance in Arabidopsis thaliana plants. Journal of Experimental Botany 58: 301–308. - PubMed
-
- Carbonell A. 2017. Plant ARGONAUTEs: features, functions, and unknowns. Methods in Molecular Biology 1640: 1–21. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical

