Phosphanylalanes and Phosphanylgallanes Stabilized only by a Lewis Base
- PMID: 31815355
- PMCID: PMC7155101
- DOI: 10.1002/anie.201914046
Phosphanylalanes and Phosphanylgallanes Stabilized only by a Lewis Base
Abstract
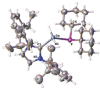
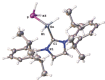
The synthesis and characterization of the first parent phosphanylalane and phosphanylgallane stabilized only by a Lewis base (LB) are reported. The corresponding substituted compounds, such as IDipp⋅GaH2 PCy2 (1) (IDipp=1,3-bis(2,6-diisopropylphenyl)-imidazolin-2-ylidene) were obtained by the reaction of LiPCy2 with IDipp⋅GaH2 Cl. However, the LB-stabilized parent compounds IDipp⋅GaH2 PH2 (3) and IDipp⋅AlH2 PH2 (4) were prepared via a salt metathesis of LiPH2 ⋅DME with IDipp⋅E'H2 Cl (E'=Ga, Al) or by H2 -elimination reactions of IDipp⋅E'H3 (E'=Ga, Al) and PH3 , respectively. The compounds could be isolated as crystalline solids and completely characterized. Supporting DFT computations gave insight into the reaction pathways as well as into the stability of these compounds with respect to their decomposition behavior.
Keywords: Lewis bases; alanes; gallanes; main-group elements; phosphorus.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- None
-
- Staubitz A., Soto A. P., Manners I., Angew. Chem. Int. Ed. 2008, 47, 6212–6215; - PubMed
- Angew. Chem. 2008, 120, 6308–6311;
-
- Clark T. J., Lee K., Manners I., Chem. Eur. J. 2006, 12, 8634–8648; - PubMed
-
- Timoshkin A. Y., Coord. Chem. Rev. 2005, 249, 2094–2131;
-
- Neumüller B., Iravani E., Coord. Chem. Rev. 2004, 248, 817–834;
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

