Optimizing Chimeric Antigen Receptor T-Cell Therapy for Adults With Acute Lymphoblastic Leukemia
- PMID: 31815579
- PMCID: PMC8312030
- DOI: 10.1200/JCO.19.01892
Optimizing Chimeric Antigen Receptor T-Cell Therapy for Adults With Acute Lymphoblastic Leukemia
Abstract
Purpose: The anti-CD19 chimeric antigen receptor T-cell therapy tisagenlecleucel (CTL019) has an 81% response rate in children with relapsed or chemotherapy refractory (r/r) B-cell acute lymphoblastic leukemia (ALL). Cytokine release syndrome (CRS) is a life-threatening treatment-related toxicity that limits the full therapeutic potential in adults. We report outcomes for adults with r/r ALL treated with an optimized CTL019 dosing and CRS management strategy.
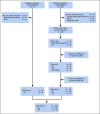
Methods: Adults with r/r B-cell ALL received CTL019 in 1 of 2 trials. Patients received lymphodepletion followed by CTL019 as either a one-time infusion or fractionated infusions split over 3 days (day 1, 10%; day 2, 30%; day 3, 60%), which allowed for day 2 and day 3 doses to be held for early CRS. Total planned CTL019 dose varied with adaptive protocol modifications in response to efficacy and CRS toxicity.
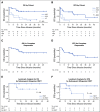
Results: Thirty-five adults with r/r ALL received CTL019 in 1 of 3 dosing cohorts. The low-dose cohort (n = 9) received single or fractionated dosing and had manageable toxicity with a 33% complete remission (CR) rate. In the high-dose single infusion cohort, 3 of 6 patients with refractory CRS concurrent with culture-positive sepsis died, and 3 achieved CR. The 20 patients in the high-dose fractionated (HDF) cohort had a 90% CR rate and manageable CRS. The HDF cohort had the highest survival, with a 2-year overall survival of 73% (95% CI, 46% to 88%) and event-free survival of 49.5% (95% CI, 21% to 73%).
Conclusion: Fractionated dosing of CTL019 with intrapatient dose modification optimizes safety without compromising efficacy in adults with r/r ALL.
Trial registration: ClinicalTrials.gov NCT02030847 NCT01029366.
Conflict of interest statement
Optimizing Chimeric Antigen Receptor T-Cell Therapy for Adults With Acute Lymphoblastic Leukemia
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Noelle V. Frey
Pamela A. Shaw
Elizabeth O. Hexner
Edward Pequignot
Saar Gill
Selina M. Luger
James K. Mangan
Alexander E. Perl
Shannon L. Maude
Stephan A. Grupp
Nirav N. Shah
Simon F. Lacey
Jos J. Melenhorst
Bruce L. Levine
Carl H. June
David L. Porter
No other potential conflicts of interest were reported.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

