Randomized, Phase II Study Prospectively Evaluating Treatment of Metastatic Esophageal, Gastric, or Gastroesophageal Cancer by Gene Expression of ERCC1: SWOG S1201
- PMID: 31815582
- PMCID: PMC7007287
- DOI: 10.1200/JCO.19.00925
Randomized, Phase II Study Prospectively Evaluating Treatment of Metastatic Esophageal, Gastric, or Gastroesophageal Cancer by Gene Expression of ERCC1: SWOG S1201
Abstract
Purpose: Platinum-based therapy is the standard of care in patients who have HER2-negative, advanced esophagogastric cancer (AEGC). Retrospective data suggest that intratumoral ERCC1 levels may determine platinum sensitivity. A randomized, phase II study was performed in patients with AEGC to explore whether the efficacy of a platinum-based therapy with fluorouracil, leucovorin, and oxaliplatin (FOLFOX) versus a non-platinum-containing regimen of irinotecan and docetaxel (IT) differed according to ERCC1 levels.
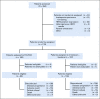
Patients and methods: Overall, 202 untreated patients with HER2-negative AEGC and a Zubrod performance status of 0-1 were evaluated prospectively for mRNA expression of ERCC1 level and then randomly assigned to FOLFOX or IT, stratified by the intratumoral statuses of ERCC1 low (< 1.7) or high (≥ 1.7). Objectives were to assess progression-free survival (PFS) and overall survival (OS) in all patients treated with FOLFOX compared with IT, stratified by low and high ERCC1 levels, and to assess for interactive effects between ERCC1 expression and treatment arm.
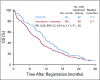
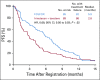
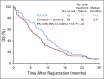
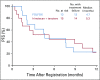
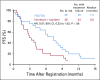
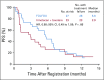
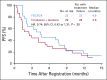
Results: Eighty-six percent of patients had ERCC1 values < 1.7. Thus, evaluation of the ERCC1-high subgroup was limited. Grade ≥ 3 anemia, dehydration, diarrhea, and fatigue were greater in patients with IT. Occurrences of grade ≥ 3 neuropathy and decreased neutrophils were greater in patients with FOLFOX. In all patients, FOLFOX had a statistically superior median PFS compared with IT (5.7 v 2.9 months; hazard ratio, 0.68; P = .02). In patients with ERCC1 levels < 1.7 receiving FOLFOX, PFS and response rate were statistically superior to IT, with no significant difference in OS.
Conclusion: The evaluation of ERCC1 in patients with upper GI tumors was thwarted by an overwhelming predominance of low ERCC1 mRNA expression. Nonetheless, distribution of treatment effects on PFS did not vary with expression. For all patients and for those with low ERCC1 expression, FOLFOX was superior in efficacy to IT.
Figures


References
-
- American Cancer Society . Cancer Facts & Figures. Atlanta, GA: American Cancer Society; 2018.
-
- Al-Batran SE, Hartmann JT, Probst S, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: A study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26:1435–1442. - PubMed
-
- Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- UG1 CA189971/CA/NCI NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- U10 CA180798/CA/NCI NIH HHS/United States
- UG1 CA189830/CA/NCI NIH HHS/United States
- UG1 CA189972/CA/NCI NIH HHS/United States
- UG1 CA189853/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- UG1 CA189957/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- U10 CA180830/CA/NCI NIH HHS/United States
- UG1 CA189804/CA/NCI NIH HHS/United States
- UG1 CA189856/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- U10 CA180801/CA/NCI NIH HHS/United States
- UG1 CA189872/CA/NCI NIH HHS/United States
- UG1 CA189822/CA/NCI NIH HHS/United States
- UG1 CA189952/CA/NCI NIH HHS/United States
- N01 CA035119/CA/NCI NIH HHS/United States
- U10 CA180846/CA/NCI NIH HHS/United States
- UG1 CA180830/CA/NCI NIH HHS/United States
- UG1 CA189858/CA/NCI NIH HHS/United States
- UG1 CA189860/CA/NCI NIH HHS/United States
- U10 CA035119/CA/NCI NIH HHS/United States
- U10 CA011083/CA/NCI NIH HHS/United States
- UG1 CA189808/CA/NCI NIH HHS/United States
- U10 CA180819/CA/NCI NIH HHS/United States
- UG1 CA189953/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

