Risk of Nephrogenic Systemic Fibrosis in Patients With Stage 4 or 5 Chronic Kidney Disease Receiving a Group II Gadolinium-Based Contrast Agent: A Systematic Review and Meta-analysis
- PMID: 31816007
- PMCID: PMC6902198
- DOI: 10.1001/jamainternmed.2019.5284
Risk of Nephrogenic Systemic Fibrosis in Patients With Stage 4 or 5 Chronic Kidney Disease Receiving a Group II Gadolinium-Based Contrast Agent: A Systematic Review and Meta-analysis
Abstract
Importance: Risk of nephrogenic systemic fibrosis (NSF) to individual patients with stage 4 or 5 chronic kidney disease (CKD; defined as estimated glomerular filtration rate of <30 mL/min/1.73 m2) who receive a group II gadolinium-based contrast agent (GBCA) is not well understood or summarized in the literature.
Objective: To assess the pooled risk of NSF in patients with stage 4 or 5 CKD receiving a group II GBCA.
Data sources: A health sciences informationist searched the Ovid (MEDLINE and MEDLINE Epub Ahead of Print, In-Process & Other Non-Indexed Citation, and Daily and Versions), Embase, Cochrane Central Register of Controlled Trials, Web of Science, and Open Grey databases from inception to January 29, 2019, yielding 2700 citations.
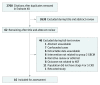
Study selection: Citations were screened for inclusion in a multistep process. Agreement for final cohort inclusion was determined by 2 blinded screeners using Cohen κ. Inclusion criteria consisted of stage 4 or 5 CKD with or without dialysis, administration of an unconfounded American College of Radiology classification group II GBCA (gadobenate dimeglumine, gadobutrol, gadoterate meglumine, or gadoteridol), and incident NSF as an outcome. Conference abstracts, retracted manuscripts, narrative reviews, editorials, case reports, and manuscripts not reporting total group II GBCA administrations were excluded.
Data extraction and synthesis: Data extraction was performed for all studies by a single investigator, including publication details, study design and time frame, patient characteristics, group II GBCA(s) administered, total exposures for patients with stage 4 or stage 5 CKD, total cases of unconfounded NSF, reason for GBCA administration, follow-up duration, loss to follow-up, basis for NSF screening, and diagnosis.
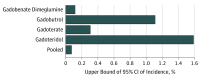
Main outcomes and measures: Pooled incidence of NSF and the associated upper bound of a 2-sided 95% CI (risk estimate) for the pooled data and each of the 4 group II GBCAs.
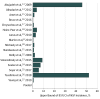
Results: Sixteen unique studies with 4931 patients were included (κ = 0.68) in this systematic review and meta-analysis. The pooled incidence of NSF was 0 of 4931 (0%; upper bound of 95% CI, 0.07%). The upper bound varied owing to different sample sizes for gadobenate dimeglumine (0 of 3167; upper bound of 95% CI, 0.12%), gadoterate meglumine (0 of 1204; upper bound of 95% CI, 0.31%), gadobutrol (0 of 330; upper bound of 95% CI, 1.11%), and gadoteridol (0 of 230; upper bound of 95% CI, 1.59%).
Conclusions and relevance: This study's findings suggest that the risk of NSF from group II GBCA administration in stage 4 or 5 CKD is likely less than 0.07%. The potential diagnostic harms of withholding group II GBCA for indicated examinations may outweigh the risk of NSF in this population.
Trial registration: PROSPERO identifier: CRD42019123284.
Conflict of interest statement
Figures
Comment in
-
Risk of Gadolinium-Based Contrast Agents in Chronic Kidney Disease-Is Zero Good Enough?JAMA Intern Med. 2020 Feb 1;180(2):230-232. doi: 10.1001/jamainternmed.2019.5278. JAMA Intern Med. 2020. PMID: 31816006 No abstract available.
-
Use of Gadolinium-Based Contrast Agents in Kidney Disease Patients: Time for Change.Am J Kidney Dis. 2020 Sep;76(3):436-439. doi: 10.1053/j.ajkd.2020.03.011. Epub 2020 Apr 10. Am J Kidney Dis. 2020. PMID: 32283121 No abstract available.
Similar articles
-
Prospective Cohort Study of Nephrogenic Systemic Fibrosis in Patients With Stage 3-5 Chronic Kidney Disease Undergoing MRI With Injected Gadobenate Dimeglumine or Gadoteridol.AJR Am J Roentgenol. 2015 Sep;205(3):469-78. doi: 10.2214/AJR.14.14268. AJR Am J Roentgenol. 2015. PMID: 26295633
-
Advancing pharmacovigilance through academic-legal collaboration: the case of gadolinium-based contrast agents and nephrogenic systemic fibrosis-a Research on Adverse Drug Events and Reports (RADAR) report.Br J Radiol. 2014 Oct;87(1042):20140307. doi: 10.1259/bjr.20140307. Br J Radiol. 2014. PMID: 25230161 Free PMC article.
-
Nephrogenic systemic fibrosis: change in incidence following a switch in gadolinium agents and adoption of a gadolinium policy--report from two U.S. universities.Radiology. 2009 Dec;253(3):689-96. doi: 10.1148/radiol.2533090649. Epub 2009 Sep 29. Radiology. 2009. PMID: 19789233
-
Adverse Events to the Gadolinium-based Contrast Agent Gadoxetic Acid: Systematic Review and Meta-Analysis.Radiology. 2020 Dec;297(3):565-572. doi: 10.1148/radiol.2020200073. Epub 2020 May 26. Radiology. 2020. PMID: 32452732
-
Gadolinium-Induced Fibrosis.Annu Rev Med. 2016;67:273-91. doi: 10.1146/annurev-med-063014-124936. Annu Rev Med. 2016. PMID: 26768242 Review.
Cited by
-
Correlation of Delayed Gadolinium-Enhanced MRI of Cartilage (dGEMRIC) Value With Hip Arthroscopy Intraoperative Findings and Midterm Periacetabular Osteotomy Outcomes.Orthop J Sports Med. 2022 Sep 2;10(9):23259671221117606. doi: 10.1177/23259671221117606. eCollection 2022 Sep. Orthop J Sports Med. 2022. PMID: 36081408 Free PMC article.
-
The Clinical Manifestations and Efficacy of Different Treatments Used for Nephrogenic Systemic Fibrosis: A Systematic Review.Int J Nephrol Renovasc Dis. 2023 Jan 12;16:17-30. doi: 10.2147/IJNRD.S392231. eCollection 2023. Int J Nephrol Renovasc Dis. 2023. PMID: 36660606 Free PMC article. Review.
-
Magnetic Resonance Imaging to Detect Cardiovascular Effects of Cancer Therapy: JACC CardioOncology State-of-the-Art Review.JACC CardioOncol. 2020 Jun 16;2(2):270-292. doi: 10.1016/j.jaccao.2020.04.011. eCollection 2020 Jun. JACC CardioOncol. 2020. PMID: 34396235 Free PMC article. Review.
-
Pharmacokinetics and Tolerability of the Cancer-Targeting MRI Contrast Agent MT218 in Healthy Males.Invest Radiol. 2024 Feb 1;59(2):165-169. doi: 10.1097/RLI.0000000000001031. Epub 2023 Dec 8. Invest Radiol. 2024. PMID: 38015107 Free PMC article. Clinical Trial.
-
Estimation of hypoperfused tissue volume in large vessel occlusions: pseudo-continuous arterial spin labeling versus dynamic susceptibility contrast perfusion-weighted imaging.Quant Imaging Med Surg. 2025 Mar 3;15(3):2053-2064. doi: 10.21037/qims-24-1560. Epub 2025 Feb 26. Quant Imaging Med Surg. 2025. PMID: 40160673 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

