Expression of FOXL2 and RSPO1 in Hen Ovarian Follicles and Implication of Exogenous Leptin in Modulating Their mRNA Expression in In Vitro Cultured Granulosa Cells
- PMID: 31817265
- PMCID: PMC6941104
- DOI: 10.3390/ani9121083
Expression of FOXL2 and RSPO1 in Hen Ovarian Follicles and Implication of Exogenous Leptin in Modulating Their mRNA Expression in In Vitro Cultured Granulosa Cells
Abstract
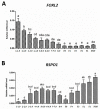
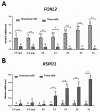
In this study, using a laying hen model, we determined the expression of FOXL2 and RSPO1 in different central and peripheral tissue and ovarian follicles at different stages of development. At the same time, mRNA expression of both genes in granulosa and theca cells harvested from follicles at different stages of folliculogenesis was also evaluated. Finally, we assessed the effect of leptin treatment on expression of FOXL2 and RSPO1 in in vitro cultured granulosa cells harvested from 1-5 mm to F3-F1 follicles. Our RT-qPCR results revealed that a comparatively higher expression of FOXL2 and RSPO1 was observed in ovary, hypothalamus, and pituitary. Abundant mRNA expression of FOXL2 was observed in small prehierarchical follicles (1-1.9 and 2-2.9 mm follicles; p < 0.05), whereas mRNA expression of RSPO1 showed an increasing trend in large hierarchical follicles (F5-F1), and its abundant expression was observed in post-ovulatory follicles. FOXL2 mRNA expression was stable in granulosa cells harvested from 3-5 mm to F4 follicles, and exhibited a significantly higher expression in large hierarchical follicles. Conversely, relatively low mRNA expression of FOXL2 was observed in theca cells. RSPO1 mRNA expression was relatively lower in granulosa cells; however, theca cells exhibited a significantly higher mRNA expression of RSPO1 in F4 to F1 follicles. In the next experiment, we treated the in vitro cultured granulosa cells with different concentrations (1, 10, 100, and 1000 ng/mL) of exogenous leptin. Compared to the control group, a significant increase in the expression of FOXL2 was observed in groups treated with 1, 10, and 100 ng/mL leptin, whereas expression of RSPO1 was increased in all leptin-treated groups. When treated with 100 ng/mL leptin, FOXL2 and RSPO1 expression was upregulated in cultured granulosa cells harvested from both large hierarchical (F3-F1) and small prehierarchical follicles (1-5 mm). Based on these findings and evidence from mainstream literature, we envisage that FOXL2 and RSPO1 genes (in connection with hypothalamic-hypophysis axis) and leptin (via modulation of FOXL2 and RSPO1 expression) might have significant physiological roles, at least in part, in modulating the ovarian mechanisms, such as follicle development, selection, and steroidogenesis in laying hens.
Keywords: FOXL2; RSPO1; granulosa cells; hierarchical follicles; laying hen; leptin; ovary; prehierarchical follicles; theca cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Attenuation by leptin of the effects of fasting on ovarian function in hens (Gallus domesticus).Reproduction. 2003 Dec;126(6):739-51. Reproduction. 2003. PMID: 14748693
-
Differential expression of mRNAs encoding the putative inhibin co-receptor (betaglycan) and activin type-I and type-II receptors in preovulatory and prehierarchical follicles of the laying hen ovary.J Endocrinol. 2006 Feb;188(2):241-9. doi: 10.1677/joe.1.06525. J Endocrinol. 2006. PMID: 16461550
-
Cooperative Effects of FOXL2 with the Members of TGF-β Superfamily on FSH Receptor mRNA Expression and Granulosa Cell Proliferation from Hen Prehierarchical Follicles.PLoS One. 2015 Oct 23;10(10):e0141062. doi: 10.1371/journal.pone.0141062. eCollection 2015. PLoS One. 2015. PMID: 26496659 Free PMC article.
-
MMPS and TIMPS in ovarian physiology and pathophysiology.Front Biosci. 2004 Sep 1;9:2474-83. doi: 10.2741/1409. Front Biosci. 2004. PMID: 15353300 Review.
-
A Bird's-Eye Overview of Leptin and Female Reproduction -with Mammalian Comparisons.J Poult Sci. 2025 Feb 6;62:2025007. doi: 10.2141/jpsa.2025007. eCollection 2025. J Poult Sci. 2025. PMID: 39916995 Free PMC article. Review.
Cited by
-
Leptin Modulates the mRNA Expression of Follicle Development Markers in Post-hatch Chicks in an Age-Dependent Manner.Front Physiol. 2021 Jul 7;12:657527. doi: 10.3389/fphys.2021.657527. eCollection 2021. Front Physiol. 2021. PMID: 34305632 Free PMC article.
-
Morphometric Evaluation of Spermatogenic Cells and Seminiferous Tubules and Exploration of Luteinizing Hormone Beta Polypeptide in Testis of Datong Yak.Animals (Basel). 2019 Dec 30;10(1):66. doi: 10.3390/ani10010066. Animals (Basel). 2019. PMID: 31905946 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

