IgA: Structure, Function, and Developability
- PMID: 31817406
- PMCID: PMC6963396
- DOI: 10.3390/antib8040057
IgA: Structure, Function, and Developability
Abstract
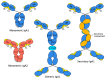
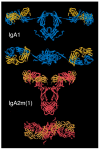
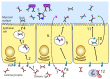
Immunoglobulin A (IgA) plays a key role in defending mucosal surfaces against attack by infectious microorganisms. Such sites present a major site of susceptibility due to their vast surface area and their constant exposure to ingested and inhaled material. The importance of IgA to effective immune defence is signalled by the fact that more IgA is produced than all the other immunoglobulin classes combined. Indeed, IgA is not just the most prevalent antibody class at mucosal sites, but is also present at significant concentrations in serum. The unique structural features of the IgA heavy chain allow IgA to polymerise, resulting in mainly dimeric forms, along with some higher polymers, in secretions. Both serum IgA, which is principally monomeric, and secretory forms of IgA are capable of neutralising and removing pathogens through a range of mechanisms, including triggering the IgA Fc receptor known as FcαRI or CD89 on phagocytes. The effectiveness of these elimination processes is highlighted by the fact that various pathogens have evolved mechanisms to thwart such IgA-mediated clearance. As the structure-function relationships governing the varied capabilities of this immunoglobulin class come into increasingly clear focus, and means to circumvent any inherent limitations are developed, IgA-based monoclonal antibodies are set to emerge as new and potent options in the therapeutic arena.
Keywords: CD89; FcαRI; IgA; immune evasion; immunoglobulin A; structure; therapeutic antibodies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
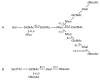
- Chintalacharuvu K.R., Raines M., Morrison S.L. Divergence of human alpha-chain constant region gene sequences. A novel recombinant alpha2 gene. J. Immunol. 1994;152:5299–5304. - PubMed
-
- Kawamura S., Saitou N., Ueda S. Concerted evolution of the primate immunoglobulin α-gene through gene conversion. J. Biol. Chem. 1992;267:7359–7367. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

