Continuous Formulation Approaches of Amorphous Solid Dispersions: Significance of Powder Flow Properties and Feeding Performance
- PMID: 31817454
- PMCID: PMC6955740
- DOI: 10.3390/pharmaceutics11120654
Continuous Formulation Approaches of Amorphous Solid Dispersions: Significance of Powder Flow Properties and Feeding Performance
Abstract
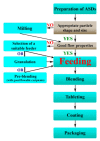
Preparation and formulation of amorphous solid dispersions (ASDs) are becoming more and more popular in the pharmaceutical field because the dissolution of poorly water-soluble drugs can be effectively improved this way, which can lead to increased bioavailability in many cases. During downstream processing of ASDs, technologists need to keep in mind both traditional challenges and the newest trends. In the last decade, the pharmaceutical industry began to display considerable interest in continuous processing, which can be explained with their potential advantages such as smaller footprint, easier scale-up, and more consistent product, better quality and quality assurance. Continuous downstream processing of drug-loaded ASDs opens new ways for automatic operation. Therefore, the formulation of poorly water-soluble drugs may be more effective and safe. However, developments can be challenging due to the poor flowability and feeding properties of ASDs. Consequently, this review pays special attention to these characteristics since the feeding of the components greatly influences the content uniformity in the final dosage form. The main purpose of this paper is to summarize the most important steps of the possible ASD-based continuous downstream processes in order to give a clear overview of current course lines and future perspectives.
Keywords: amorphous solid dispersions; continuous manufacturing; feeding; formulation; powder characterization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Borbás E., Sinkó B.L., Tsinman O., Tsinman K., Kiserdei E.V., Démuth B.Z., Balogh A., Bodák B., Domokos A.S., Dargó G. Investigation and mathematical description of the real driving force of passive transport of drug molecules from supersaturated solutions. Mol. Pharm. 2016;13:3816–3826. doi: 10.1021/acs.molpharmaceut.6b00613. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

