Dihydrochalcones: Methods of Acquisition and Pharmacological Properties-A First Systematic Review
- PMID: 31817526
- PMCID: PMC6943545
- DOI: 10.3390/molecules24244468
Dihydrochalcones: Methods of Acquisition and Pharmacological Properties-A First Systematic Review
Abstract
Dihydrochalcones are a class of secondary metabolites, for which demand in biological and pharmacological applications is still growing. They posses several healthendorsing properties and, therefore, are promising candidates for further research and development. However, low content of dihydrochalcones in plants along with their low solubility and bioavailability restrict the development of these compounds as clinical therapeutics. Therefore, chemomicrobial and enzymatic modifications are required to expand their application. This review aims at analyzing and summarizing the methods of obtaining dihydrochalcones and of presenting their pharmacological actions that have been described in the literature to support potential future development of this group of compounds as novel therapeutic drugs. We have also performed an evaluation of the available literature on beneficial effects of dihydrochalcones with potent antioxidant activity and multifactorial pharmacological effects, including antidiabetic, antitumor, lipometabolism regulating, antioxidant, antiinflammatory, antibacterial, antiviral, and immunomodulatory ones. In addition, we provide useful information on their properties, sources, and usefulness in medicinal chemistry.
Keywords: antioxidants; dihydrochalcones; microbial transformations; phloretin; sweeteners.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



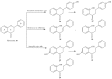




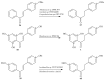
References
-
- Yan W., Li B., Li R. Solubilities of phloretin in 12 solvents at different temperatures. J. Chem. Eng. Data. 2011;56:1459–1462.
-
- Tang N., Yan W. Solubilities of naringin dihydrochalcone in pure solvents and mixed solvents at different temperatures. J. Chem. Eng. Data. 2016;61:4085–4089. doi: 10.1021/acs.jced.6b00543. - DOI
-
- Kim M., Shin B.-K., Won D., Han J. Synthesis of 2′-hydroxydihydrochalcone from flavone. J. Appl. Biol. Chem. 2007;50:85–87.
-
- Semalty A., Semalty M., Singh D., Rawat M.S.M. Preparation and characterization of phospholipid complexes of naringenin for effective drug delivery. J. Incl. Phenom. Macrocycl. Chem. 2010;67:253–260. doi: 10.1007/s10847-009-9705-8. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

