PEGylated Purpurin 18 with Improved Solubility: Potent Compounds for Photodynamic Therapy of Cancer
- PMID: 31817655
- PMCID: PMC6943672
- DOI: 10.3390/molecules24244477
PEGylated Purpurin 18 with Improved Solubility: Potent Compounds for Photodynamic Therapy of Cancer
Abstract
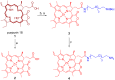
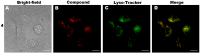
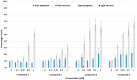
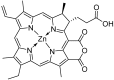
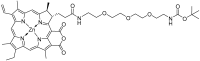
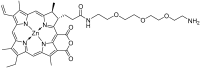
Purpurin 18 derivatives with a polyethylene glycol (PEG) linker were synthesized as novel photosensitizers (PSs) with the goal of using them in photodynamic therapy (PDT) for cancer. These compounds, derived from a second-generation PS, exhibit absorption at long wavelengths; considerable singlet oxygen generation and, in contrast to purpurin 18, have higher hydrophilicity due to decreased logP. Together, these properties make them potentially ideal PSs. To verify this, we screened the developed compounds for cell uptake, intracellular localization, antitumor activity and induced cell death type. All of the tested compounds were taken up into cancer cells of various origin and localized in organelles known to be important PDT targets, specifically, mitochondria and the endoplasmic reticulum. The incorporation of a zinc ion and PEGylation significantly enhanced the photosensitizing efficacy, decreasing IC50 (half maximal inhibitory compound concentration) in HeLa cells by up to 170 times compared with the parental purpurin 18. At effective PDT concentrations, the predominant type of induced cell death was apoptosis. Overall, our results show that the PEGylated derivatives presented have significant potential as novel PSs with substantially augmented phototoxicity for application in the PDT of cervical, prostate, pancreatic and breast cancer.
Keywords: PEGylated purpurin 18; apoptosis; cancer cells; cytotoxicity; flow cytometry; live-cell fluorescence microscopy; photodynamic therapy; photosensitizer; phototoxicity; singlet oxygen.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
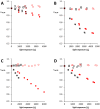

 —Solution exposed to light,
—Solution exposed to light, 
 —Solution kept in dark. crel,AB—Relative concentration of AB (actual concentration with respect to concentration at experiment start).
—Solution kept in dark. crel,AB—Relative concentration of AB (actual concentration with respect to concentration at experiment start).



References
-
- Tang P.M., Chan J.Y., Au S.W., Kong S.K., Tsui S.K., Waye M.M., Mak T.C., Fong W.P., Fung K.P. Pheophorbide a, an active compound isolated from Scutellaria barbata, possesses photodynamic activities by inducing apoptosis in human hepatocellular carcinoma. Cancer Biol. Ther. 2006;5:1111–1116. doi: 10.4161/cbt.5.9.2950. - DOI - PubMed
-
- Pavlíčková V., Jurášek M., Rimpelová S., Záruba K., Sedlák D., Šimková M., Kodr D., Staňková E., Fähnrich J., Rottnerová Z., et al. Oxime-based 19-nortestosterone–pheophorbide a conjugate: Bimodal controlled release concept for PDT. J. Mater. Chem. B. 2019;7:5465–5477. doi: 10.1039/C9TB01301F. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

