Targeting mTOR and Metabolism in Cancer: Lessons and Innovations
- PMID: 31817676
- PMCID: PMC6952948
- DOI: 10.3390/cells8121584
Targeting mTOR and Metabolism in Cancer: Lessons and Innovations
Abstract
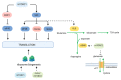
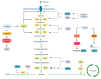
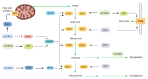
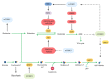
Cancer cells support their growth and proliferation by reprogramming their metabolism in order to gain access to nutrients. Despite the heterogeneity in genetic mutations that lead to tumorigenesis, a common alteration in tumors occurs in pathways that upregulate nutrient acquisition. A central signaling pathway that controls metabolic processes is the mTOR pathway. The elucidation of the regulation and functions of mTOR can be traced to the discovery of the natural compound, rapamycin. Studies using rapamycin have unraveled the role of mTOR in the control of cell growth and metabolism. By sensing the intracellular nutrient status, mTOR orchestrates metabolic reprogramming by controlling nutrient uptake and flux through various metabolic pathways. The central role of mTOR in metabolic rewiring makes it a promising target for cancer therapy. Numerous clinical trials are ongoing to evaluate the efficacy of mTOR inhibition for cancer treatment. Rapamycin analogs have been approved to treat specific types of cancer. Since rapamycin does not fully inhibit mTOR activity, new compounds have been engineered to inhibit the catalytic activity of mTOR to more potently block its functions. Despite highly promising pre-clinical studies, early clinical trial results of these second generation mTOR inhibitors revealed increased toxicity and modest antitumor activity. The plasticity of metabolic processes and seemingly enormous capacity of malignant cells to salvage nutrients through various mechanisms make cancer therapy extremely challenging. Therefore, identifying metabolic vulnerabilities in different types of tumors would present opportunities for rational therapeutic strategies. Understanding how the different sources of nutrients are metabolized not just by the growing tumor but also by other cells from the microenvironment, in particular, immune cells, will also facilitate the design of more sophisticated and effective therapeutic regimen. In this review, we discuss the functions of mTOR in cancer metabolism that have been illuminated from pre-clinical studies. We then review key findings from clinical trials that target mTOR and the lessons we have learned from both pre-clinical and clinical studies that could provide insights on innovative therapeutic strategies, including immunotherapy to target mTOR signaling and the metabolic network in cancer.
Keywords: cancer metabolism; kinase inhibitors; mTOR in immunotherapy; mTOR inhibitors; mTOR signaling; mTORC1; mTORC2; metabolism; nutrient metabolism; rapalogs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

