Review of the Quench Sensitivity of Aluminium Alloys: Analysis of the Kinetics and Nature of Quench-Induced Precipitation
- PMID: 31817746
- PMCID: PMC6947292
- DOI: 10.3390/ma12244083
Review of the Quench Sensitivity of Aluminium Alloys: Analysis of the Kinetics and Nature of Quench-Induced Precipitation
Abstract
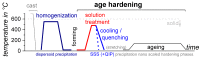
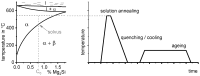
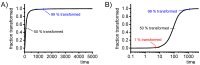
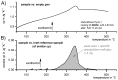
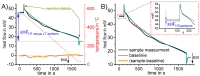
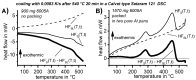
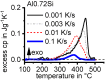
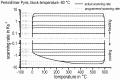
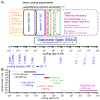
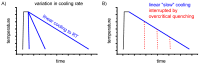
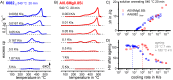
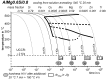
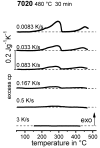
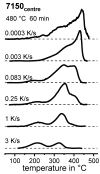
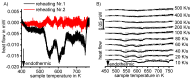
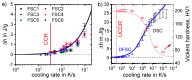
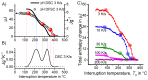
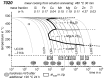
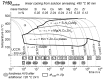
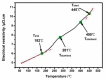
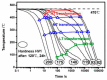
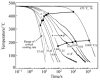
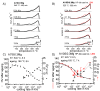
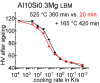
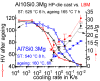
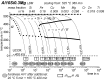
For aluminium alloys, precipitation strengthening is controlled by age-hardening heat treatments, including solution treatment, quenching, and ageing. In terms of technological applications, quenching is considered a critical step, because detrimental quench-induced precipitation must be avoided to exploit the full age-hardening potential of the alloy. The alloy therefore needs to be quenched faster than a critical cooling rate, but slow enough to avoid undesired distortion and residual stresses. These contrary requirements for quenching can only be aligned based on detailed knowledge of the kinetics of quench-induced precipitation. Until the beginning of the 21st century, the kinetics of relevant solid-solid phase transformations in aluminium alloys could only be estimated by ex-situ testing of different properties. Over the past ten years, significant progress has been achieved in this field of materials science, enabled by the development of highly sensitive differential scanning calorimetry (DSC) techniques. This review presents a comprehensive report on the solid-solid phase transformation kinetics in Al alloys covering precipitation and dissolution reactions during heating from different initial states, dissolution during solution annealing and to a vast extent quench-induced precipitation during continuous cooling over a dynamic cooling rate range of ten orders of magnitude. The kinetic analyses are complemented by sophisticated micro- and nano-structural analyses and continuous cooling precipitation (CCP) diagrams are derived. The measurement of enthalpies released by quench-induced precipitation as a function of the cooling rate also enables predictions of the quench sensitivities of Al alloys using physically-based models. Various alloys are compared, and general aspects of quench-induced precipitation in Al alloys are derived.
Keywords: AlCu wrought alloys; AlMgSi wrought alloys; AlSi wrought alloys; AlSiMg cast alloys; AlZnMg wrought alloys; DSC; aluminium alloys; kinetics; quench induces precipitation; quench sensitivity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






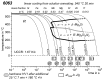
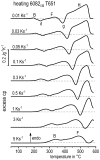
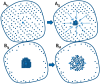
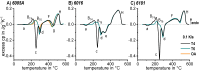
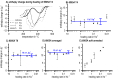
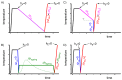
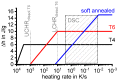
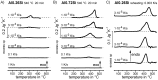
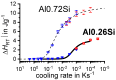



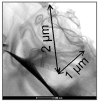



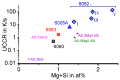








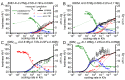
















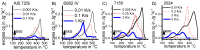
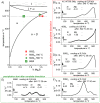

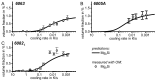
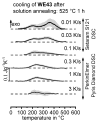
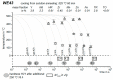
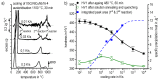
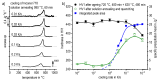
References
-
- Statista Global Raw-Steel Production from 2002 to 2017. ID153008, Recource: World Steel Association. [(accessed on 11 July 2018)]; Available online: https://de.statista.com/statistik/daten/studie/153008/umfrage/welt-rohst...
-
- Statista Global Production Amount of Major non-Ferrus Metals in 2013 and 2014. 323132 Resource: World Bureau of Metal Statistics. [(accessed on 11 July 2018)]; Available online: https://de.statista.com/statistik/daten/studie/323132/umfrage/produktion...
-
- Statista . American Foundry Society; [(accessed on 11 July 2018)]. Global Production of Castings from Various Metals in 2016. Ressources: European Foundry Association. Available online: https://de.statista.com/statistik/daten/studie/421534/umfrage/produktion...
-
- Statista Global Demand for Aluminum from 2006 to 2019. ID156039. [(accessed on 11 July 2018)]; Available online: https://de.statista.com/statistik/daten/studie/156039/umfrage/weltweite-...
-
- Polmear I.J. Light Alloys from Traditional Alloys to Nanocrystals. 4th ed. Elsevier Butterworth-Heinemann; Amsterdam, The Netherlands: 2006.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

