Induction of NK Cell Reactivity against B-Cell Acute Lymphoblastic Leukemia by an Fc-Optimized FLT3 Antibody
- PMID: 31817795
- PMCID: PMC6966676
- DOI: 10.3390/cancers11121966
Induction of NK Cell Reactivity against B-Cell Acute Lymphoblastic Leukemia by an Fc-Optimized FLT3 Antibody
Abstract
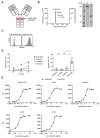
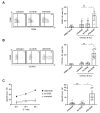
Antibody-dependent cellular cytotoxicity (ADCC) is a major mechanism by which antitumor antibodies mediate therapeutic efficacy. At present, we evaluate an Fc-optimized (amino acid substitutions S239D/I332E) FLT3 antibody termed 4G8-SDIEM (FLYSYN) in patients with acute myeloid leukemia (NCT02789254). Here we studied the possibility to induce NK cell ADCC against B-cell acute lymphoblastic leukemia (B-ALL) by Fc-optimized FLT3 antibody treatment. Flow cytometric analysis confirmed that FLT3 is widely expressed on B-ALL cell lines and leukemic cells of B-ALL patients. FLT3 expression did not correlate with that of CD20, which is targeted by Rituximab, a therapeutic monoclonal antibody (mAb) employed in B-ALL treatment regimens. Our FLT3 mAb with enhanced affinity to the Fc receptor CD16a termed 4G8-SDIE potently induced NK cell reactivity against FLT3-transfectants, the B-ALL cell line SEM and primary leukemic cells of adult B-ALL patients in a target-antigen dependent manner as revealed by analyses of NK cell activation and degranulation. This was mirrored by potent 4G8-SDIE mediated NK cell ADCC in experiments with FLT3-transfectants, the cell line SEM and primary cells as target cells. Taken together, the findings presented in this study provide evidence that 4G8-SDIE may be a promising agent for the treatment of B-ALL, particularly in CD20-negative cases.
Keywords: ADCC; B-ALL; CD135; FLT3; NK cells; acute lymphoblastic leukemia; antibody; immunotherapy.
Conflict of interest statement
H.-J.B. and G.J. were listed as inventors in a patent family for Fc-optimized FLT3 mAb, e.g., EP2516468B1; applicant was Tübingen University. There are no other conflicts of interest to declare.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

