Role of MyD88 in IL-1β and Ethanol Modulation of GABAergic Transmission in the Central Amygdala
- PMID: 31817854
- PMCID: PMC6956324
- DOI: 10.3390/brainsci9120361
Role of MyD88 in IL-1β and Ethanol Modulation of GABAergic Transmission in the Central Amygdala
Abstract
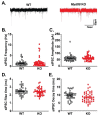
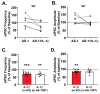
Myeloid differentiation primary response protein (MyD88) is a critical neuroimmune adaptor protein in TLR (Toll-like receptor) and IL-1R (Interleukin-1 receptor) signaling complexes. These two pro-inflammatory families play an important role in the neurobiology of alcohol use disorder, specifically MyD88 regulates ethanol drinking, ethanol-induced sedation, and ethanol-induced deficits in motor coordination. In this study, we examined the role of MyD88 in mediating the effects of IL-1β and ethanol on GABAergic transmission in the central amygdala (CeA) of male mice using whole-cell patch-clamp recordings in combination with pharmacological (AS-1, a mimetic that prevents MyD88 recruitment by IL-1R) and genetic (Myd88 knockout mice) approaches. We demonstrate through both approaches that IL-1β and ethanol's modulatory effects at CeA GABA synapses are not dependent on MyD88. Myd88 knockout potentiated IL-1β's actions in reducing postsynaptic GABAA receptor function. Pharmacological inhibition of MyD88 modulates IL-1β's action at CeA GABA synapses similar to Myd88 knockout mice. Additionally, ethanol-induced CeA GABA release was greater in Myd88 knockout mice compared to wildtype controls. Thus, MyD88 is not essential to IL-1β or ethanol regulation of CeA GABA synapses but plays a role in modulating the magnitude of their effects, which may be a potential mechanism by which it regulates ethanol-related behaviors.
Keywords: GABA; IL-1R1; Myd88 knockout; alcohol; interleukin-1; neuroimmune; sIPSC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Mulligan M.K., Ponomarev I., Hitzemann R.J., Belknap J.K., Tabakoff B., Harris R.A., Crabbe J.C., Blednov Y.A., Grahame N.J., Phillips T.J., et al. Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc. Natl. Acad. Sci. USA. 2006;103:6368–6373. doi: 10.1073/pnas.0510188103. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

