Bioresorbable Vascular Scaffolds-Dead End or Still a Rough Diamond?
- PMID: 31817876
- PMCID: PMC6947479
- DOI: 10.3390/jcm8122167
Bioresorbable Vascular Scaffolds-Dead End or Still a Rough Diamond?
Abstract
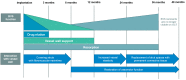
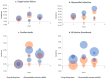
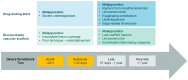
Percutaneous coronary interventions with stent-based restorations of vessel patency have become the gold standard in the treatment of acute coronary states. Bioresorbable vascular scaffolds (BVS) have been designed to combine the efficiency of drug-eluting stents (DES) at the time of implantation and the advantages of a lack of foreign body afterwards. Complete resolution of the scaffold was intended to enable the restoration of vasomotor function and reduce the risk of device thrombosis. While early reports demonstrated superiority of BVS over DES, larger-scale application and longer observation exposed major concerns about their use, including lower radial strength and higher risk of thrombosis resulting in higher rate of major adverse cardiac events. Further focus on procedural details and research on the second generation of BVS with novel properties did not allow to unequivocally challenge position of DES. Nevertheless, BVS still have a chance to present superiority in distinctive indications. This review presents an outlook on the available first and second generation BVS and a summary of results of clinical trials on their use. It discusses explanations for unfavorable outcomes, proposed enhancement techniques and a potential niche for the use of BVS.
Keywords: acute coronary syndrome; angioplasty; bioresorbable vascular scaffold; drug-eluting stent; percutaneous coronary intervention.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Vahl T.P., Gasior P., Gongora C.A., Ramzipoor K., Lee C., Cheng Y. Four-year polymer biocompatibility and vascular healing profile of a novel ultrahigh molecular weight amorphous PLLA bioresorbable vascular scaffold: An OCT study in healthy porcine coronary arteries. EuroIntervention. 2016;12:1510–1518. doi: 10.4244/EIJ-D-16-00308. - DOI - PubMed
-
- Gonzalo N., Otaegui I., Rumoroso J.R., Gutiérrez H., Alfonso F., Marti G. Device specificity of vascular healing following implantation of bioresorbable vascular scaffolds and bioabsorbable polymer metallic drug-eluting stents in human coronary arteries: The ESTROFA OCT BVS vs. BP-DES study. EuroIntervention. 2018;14:e1295–e1303. doi: 10.4244/eij-d-17-00952. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

