Magnetic Nanoparticles Supporting Bio-responsive T1/ T2 Magnetic Resonance Imaging
- PMID: 31817929
- PMCID: PMC6947368
- DOI: 10.3390/ma12244096
Magnetic Nanoparticles Supporting Bio-responsive T1/ T2 Magnetic Resonance Imaging
Abstract
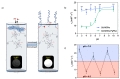
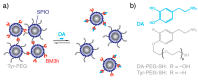
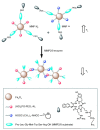
: The use of nanoparticulate systems as contrast agents for magnetic resonance imaging (MRI) is well-established and known to facilitate an enhanced image sensitivity within scans of a particular pathological region of interest. Such a capability can enable both a non-invasive diagnosis and the monitoring of disease progression/response to treatment. In this review, magnetic nanoparticles that exhibit a bio-responsive MR relaxivity are discussed, with pH-, enzyme-, biomolecular-, and protein-responsive systems considered. The ability of a contrast agent to respond to a biological stimulus provides not only enriched diagnostic capabilities over corresponding non-responsive analogues, but also an improved longitudinal monitoring of specific physiological conditions.
Keywords: Bio-responsive; Biomolecule-responsive; Diagnosis; Enzyme-responsive; Iron Oxide Nanoparticles; Magnetic Resonance Imaging; Mesoporous Silica Nanoparticles; Nanoparticle; Therapy; pH-responsive.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

