Noncoding RNAs and Liquid Biopsy in Lung Cancer: A Literature Review
- PMID: 31818027
- PMCID: PMC6963838
- DOI: 10.3390/diagnostics9040216
Noncoding RNAs and Liquid Biopsy in Lung Cancer: A Literature Review
Abstract
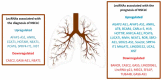
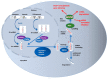
Lung cancer represents a genetically heterogeneous disease with low survival rates. Recent data have evidenced key roles of noncoding RNAs in lung cancer initiation and progression. These functional RNA molecules that can act as both oncogenes and tumor suppressors may become future biomarkers and more efficient therapeutic targets. In the precision medicine era, circulating nucleic acids have the potential to reshape the management and prognosis of cancer patients. Detecting genomic alterations and level variations of circulating nucleic acids in liquid biopsy samples represents a noninvasive method for portraying tumor burden. Research is currently trying to validate the potential role of liquid biopsy in lung cancer screening, prognosis, monitoring of disease progression, and treatment response. However, this method requires complex detection assays, and implementation of plasma genotyping in clinical practice continues to be hindered by discrepancies that arise when compared to tissue genotyping. Understanding the genomic landscape of lung cancer is essential in order to provide useful and innovative research in the age of patient-tailored therapy. In this landscape, the noncoding RNAs play a crucial role due to their target genes that dramatically influence the tumor microenvironment and the response to therapy. This article addresses present and future possible roles of liquid biopsy in lung cancer. It also discusses how the complex role of noncoding RNAs in lung tumorigenesis could influence the management of this pathology.
Keywords: liquid biopsy; lung cancer; noncoding RNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

