Augmented Radiologist Workflow Improves Report Value and Saves Time: A Potential Model for Implementation of Artificial Intelligence
- PMID: 31818390
- PMCID: PMC8189646
- DOI: 10.1016/j.acra.2019.09.014
Augmented Radiologist Workflow Improves Report Value and Saves Time: A Potential Model for Implementation of Artificial Intelligence
Abstract
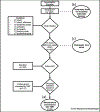
Rationale and objectives: Our primary aim was to improve radiology reports by increasing concordance of target lesion measurements with oncology records using radiology preprocessors (RP). Faster notification of incidental actionable findings to referring clinicians and clinical radiologist exam interpretation time savings with RPs quantifying tumor burden were also assessed.
Materials and methods: In this prospective quality improvement initiative, RPs annotated lesions before radiologist interpretation of CT exams. Clinical radiologists then hyperlinked approved measurements into interactive reports during interpretations. RPs evaluated concordance with our tumor measurement radiologist, the determinant of tumor burden. Actionable finding detection and notification times were also deduced. Clinical radiologist interpretation times were calculated from established average CT chest, abdomen, and pelvis interpretation times.
Results: RPs assessed 1287 body CT exams with 812 follow-up CT chest, abdomen, and pelvis studies; 95 (11.7%) of which had 241 verified target lesions. There was improved concordance (67.8% vs. 22.5%) of target lesion measurements. RPs detected 93.1% incidental actionable findings with faster clinician notification by a median time of 1 hour (range: 15 minutes-16 hours). Radiologist exam interpretation times decreased by 37%.
Conclusions: This workflow resulted in three-fold improved target lesion measurement concordance with oncology records, earlier detection and faster notification of incidental actionable findings to referring clinicians, and decreased exam interpretation times for clinical radiologists. These findings demonstrate potential roles for automation (such as AI) to improve report value, worklist prioritization, and patient care.
Keywords: Actionable findings; Artificial intelligence; Cancer clinical trials; Radiology preprocessors; Tumor quantification.
Published by Elsevier Inc.
Conflict of interest statement
DECLARATION OF INTEREST
None declared.
CONFLICT OF INTEREST
Dr. Huy Do and Dr. Les Folio were associate investigators in a corporate research agreement with Carestream Health (Rochester, NY), the PACS used in this initiative.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

