The Arabidopsis At GCD3 protein is a glucosylceramidase that preferentially hydrolyzes long-acyl-chain glucosylceramides
- PMID: 31819005
- PMCID: PMC6970933
- DOI: 10.1074/jbc.RA119.011274
The Arabidopsis At GCD3 protein is a glucosylceramidase that preferentially hydrolyzes long-acyl-chain glucosylceramides
Abstract
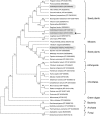
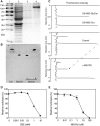
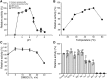
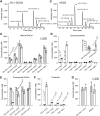
Cellular membranes contain many lipids, some of which, such as sphingolipids, have important structural and signaling functions. The common sphingolipid glucosylceramide (GlcCer) is present in plants, fungi, and animals. As a major plant sphingolipid, GlcCer is involved in the formation of lipid microdomains, and the regulation of GlcCer is key for acclimation to stress. Although the GlcCer biosynthetic pathway has been elucidated, little is known about GlcCer catabolism, and a plant GlcCer-degrading enzyme (glucosylceramidase (GCD)) has yet to be identified. Here, we identified AtGCD3, one of four Arabidopsis thaliana homologs of human nonlysosomal glucosylceramidase, as a plant GCD. We found that recombinant AtGCD3 has a low Km for the fluorescent lipid C6-NBD GlcCer and preferentially hydrolyzes long acyl-chain GlcCer purified from Arabidopsis leaves. Testing of inhibitors of mammalian glucosylceramidases revealed that a specific inhibitor of human β-glucosidase 2, N-butyldeoxynojirimycin, inhibits AtGCD3 more effectively than does a specific inhibitor of human β-glucosidase 1, conduritol β-epoxide. We also found that Glu-499 and Asp-647 in AtGCD3 are vital for GCD activity. GFP-AtGCD3 fusion proteins mainly localized to the plasma membrane or the endoplasmic reticulum membrane. No obvious growth defects or changes in sphingolipid contents were observed in gcd3 mutants. Our results indicate that AtGCD3 is a plant glucosylceramidase that participates in GlcCer catabolism by preferentially hydrolyzing long-acyl-chain GlcCers.
Keywords: Arabidopsis thaliana; cell signaling; ceramide; fatty acid; glucosylceramidase; lipid metabolism; plant biology; sphingolipid; stress adaption.
© 2020 Dai et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


Similar articles
-
A potential pathway for flippase-facilitated glucosylceramide catabolism in plants.Plant Signal Behav. 2020 Oct 2;15(10):1783486. doi: 10.1080/15592324.2020.1783486. Epub 2020 Aug 28. Plant Signal Behav. 2020. PMID: 32857675 Free PMC article.
-
Sphingolipid remodeling in the plasma membrane is essential for osmotic stress tolerance in Arabidopsis.Plant Physiol. 2025 Feb 7;197(2):kiaf031. doi: 10.1093/plphys/kiaf031. Plant Physiol. 2025. PMID: 39843213
-
Glucosylceramides impact cellulose deposition and cellulose synthase complex motility in Arabidopsis.Glycobiology. 2024 Apr 24;34(6):cwae035. doi: 10.1093/glycob/cwae035. Glycobiology. 2024. PMID: 38690785
-
The metabolism of glucocerebrosides - From 1965 to the present.Mol Genet Metab. 2017 Jan-Feb;120(1-2):22-26. doi: 10.1016/j.ymgme.2016.11.390. Epub 2016 Dec 2. Mol Genet Metab. 2017. PMID: 27955980 Review.
-
New insights on glucosylated lipids: metabolism and functions.Biochim Biophys Acta. 2013 Sep;1831(9):1475-85. doi: 10.1016/j.bbalip.2013.06.001. Epub 2013 Jun 13. Biochim Biophys Acta. 2013. PMID: 23770033 Review.
Cited by
-
A potential pathway for flippase-facilitated glucosylceramide catabolism in plants.Plant Signal Behav. 2020 Oct 2;15(10):1783486. doi: 10.1080/15592324.2020.1783486. Epub 2020 Aug 28. Plant Signal Behav. 2020. PMID: 32857675 Free PMC article.
-
Plasma and vacuolar membrane sphingolipidomes: composition and insights on the role of main molecular species.Plant Physiol. 2021 May 27;186(1):624-639. doi: 10.1093/plphys/kiab064. Plant Physiol. 2021. PMID: 33570616 Free PMC article.
-
Novel SNP markers for flowering and seed quality traits in faba bean (Vicia faba L.): characterization and GWAS of a diversity panel.Front Plant Sci. 2024 Mar 6;15:1348014. doi: 10.3389/fpls.2024.1348014. eCollection 2024. Front Plant Sci. 2024. PMID: 38510437 Free PMC article.
-
Sphingolipid metabolism, transport, and functions in plants: Recent progress and future perspectives.Plant Commun. 2021 Jun 29;2(5):100214. doi: 10.1016/j.xplc.2021.100214. eCollection 2021 Sep 13. Plant Commun. 2021. PMID: 34746760 Free PMC article. Review.
-
Selective labelling of GBA2 in cells with fluorescent β-d-arabinofuranosyl cyclitol aziridines.Chem Sci. 2024 Sep 3;15(37):15212-20. doi: 10.1039/d3sc06146a. Online ahead of print. Chem Sci. 2024. PMID: 39246358 Free PMC article.
References
-
- Bi F.-C., Liu Z., Wu J.-X., Liang H., Xi X.-L., Fang C., Sun T.-J., Yin J., Dai G.-Y., Rong C., Greenberg J. T., Su W.-W., and Yao N. (2014) Loss of ceramide kinase in Arabidopsis impairs defenses and promotes ceramide accumulation and mitochondrial H2O2 bursts. Plant Cell 26, 3449–3467 10.1105/tpc.114.127050 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

