The Arabidopsis At GCD3 protein is a glucosylceramidase that preferentially hydrolyzes long-acyl-chain glucosylceramides
- PMID: 31819005
- PMCID: PMC6970933
- DOI: 10.1074/jbc.RA119.011274
The Arabidopsis At GCD3 protein is a glucosylceramidase that preferentially hydrolyzes long-acyl-chain glucosylceramides
Abstract
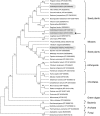
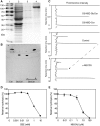
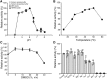
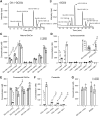
Cellular membranes contain many lipids, some of which, such as sphingolipids, have important structural and signaling functions. The common sphingolipid glucosylceramide (GlcCer) is present in plants, fungi, and animals. As a major plant sphingolipid, GlcCer is involved in the formation of lipid microdomains, and the regulation of GlcCer is key for acclimation to stress. Although the GlcCer biosynthetic pathway has been elucidated, little is known about GlcCer catabolism, and a plant GlcCer-degrading enzyme (glucosylceramidase (GCD)) has yet to be identified. Here, we identified AtGCD3, one of four Arabidopsis thaliana homologs of human nonlysosomal glucosylceramidase, as a plant GCD. We found that recombinant AtGCD3 has a low Km for the fluorescent lipid C6-NBD GlcCer and preferentially hydrolyzes long acyl-chain GlcCer purified from Arabidopsis leaves. Testing of inhibitors of mammalian glucosylceramidases revealed that a specific inhibitor of human β-glucosidase 2, N-butyldeoxynojirimycin, inhibits AtGCD3 more effectively than does a specific inhibitor of human β-glucosidase 1, conduritol β-epoxide. We also found that Glu-499 and Asp-647 in AtGCD3 are vital for GCD activity. GFP-AtGCD3 fusion proteins mainly localized to the plasma membrane or the endoplasmic reticulum membrane. No obvious growth defects or changes in sphingolipid contents were observed in gcd3 mutants. Our results indicate that AtGCD3 is a plant glucosylceramidase that participates in GlcCer catabolism by preferentially hydrolyzing long-acyl-chain GlcCers.
Keywords: Arabidopsis thaliana; cell signaling; ceramide; fatty acid; glucosylceramidase; lipid metabolism; plant biology; sphingolipid; stress adaption.
© 2020 Dai et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Bi F.-C., Liu Z., Wu J.-X., Liang H., Xi X.-L., Fang C., Sun T.-J., Yin J., Dai G.-Y., Rong C., Greenberg J. T., Su W.-W., and Yao N. (2014) Loss of ceramide kinase in Arabidopsis impairs defenses and promotes ceramide accumulation and mitochondrial H2O2 bursts. Plant Cell 26, 3449–3467 10.1105/tpc.114.127050 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

